A hydrogel to prevent fracture-related infection
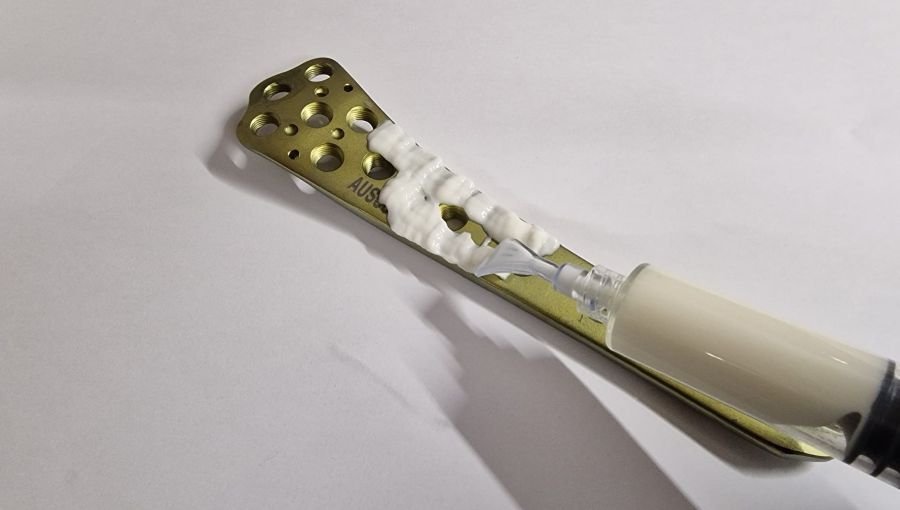
Fracture-related infection (FRI) is a devastating complication following bone fractures. It can result in prolonged recovery times, mal- or non-union, multiple revision surgeries, lifelong functional impairment, and leads to dramatically elevated costs.
GEDAI-T (hydroGEl for Delivery of AntIbiotics, using Tobramycin), a new, bioresorbable, injectable, antibiotic-eluting hydrogel targeting the prevention of FRI, was developed by AO Research Institute Davos (ARI) to deliver high doses of antibiotics to the injury site.
Prevention and treatment of infection following bone fractures and osteosynthesis go hand in hand since antibiotics can provide both. Traditionally, antibiotics have been provided systemically—oral, intravenous, or through intramuscular injection. “The problem with systemic administration is that if you exceed a certain level, a certain tissue or blood serum concentration, you enter the toxic spectrum, and human cells will be affected by the antibiotic as well,” explained Michiel Verhofstad, Professor of Trauma Surgery at Erasmus MC in Rotterdam, the Netherlands, who has been involved in the development of GEDAI-T as the chairperson of the AO Technical Commission’s Anti Infection Global Expert Committee. “However, if the dose is too low, bacteria will adapt to the new situation, and those less susceptible to the antibiotic will survive.”
Verhofstad sees clear advantages in local application: “When you apply antibiotics locally, you need much less to get the same concentration in the field of interest. And if you use the same amount in local application as you would in systemic treatment, you can achieve a much higher local concentration without the systemic side effects. If you have a high concentration, bacteria which are considered resistant to a certain antibiotic may become susceptible again.
However, the “how to” of applying antibiotics locally is an important question. Antibiotic powders added to the fracture site before the wound is closed will result in short-term high peaks and may spread throughout the body, eventually acting systemically. “What I, as a surgeon, would like to have is a dissolving carrier that contains the antibiotic and gradually releases it—and a gel would be my first choice,” said Verhofstad.
The formulation of GEDAI-T was developed by Matteo D’Este and the Biomedical Materials team at ARI, following specific requirements to its handling properties provided by surgeons from within the AO’s network, providing a highly versatile platform with the potential of bringing clinical benefit well beyond the prevention and treatment of FRI. It is one of several activities in the focus of ARI’s Infection Biology team under the lead of Fintan Moriarty, aimed at better understanding FRI to provide better treatments and improve clinical outcomes.
Antibiotic powders have been used off-label in the United States for a while now to treat and prevent FRI, but there is no gel on the market for this purpose, although a survey we did showed that it would be surgeons’ preferred manner of delivering antibiotics,” said Pamela Nylund, who is the Project Leader for the development of GEDAI-T at ARI. “The gel is very versatile and isn’t limited to fracture type or location.” Although the gel is currently being tested and approved with only one antibiotic—tobramycin—it could potentially carry and release other antibiotics and chemical substances in the future.
Over the following months, the gel will be applied in animal studies performed at ARI’s pre-clinical facilities to demonstrate its safety and efficacy. “We are looking at the effect on fracture healing, efficacy, and local and systemic toxicology,” said Andrea Montali, Manager of the AO’s Development Incubator. “We’re expecting to start the first clinical trials on humans in 2026 and are currently seeking industrial partners.” Following the initial development, which was conducted at ARI, the translational phase of the project is being funded and supported by AO’s innovation funding through its Development Incubator with the goal of compiling the necessary data, documentation and IP to make this an attractive business opportunity for a future industrial partner.
Although it will still take a few years for GEDAI-T to be fully approved, regulated, and market-ready, Verhofstad is confident it will eventually become a successful product: ”The gel has great potential. It is based on readily available and well-known raw materials with a long track record of use in the pharmaceutical and medical industries, and it dissolves gradually without leaving any footprint. The question is whether I would consider volunteering in a clinical study, and yes, I would.”
GEDAI-T (hydroGEl for Delivery of AntIbiotics, using Tobramycin), a new, bioresorbable, injectable, antibiotic-eluting hydrogel targeting the prevention of FRI, was developed by AO Research Institute Davos (ARI) to deliver high doses of antibiotics to the injury site.
Prevention and treatment of infection following bone fractures and osteosynthesis go hand in hand since antibiotics can provide both. Traditionally, antibiotics have been provided systemically—oral, intravenous, or through intramuscular injection. “The problem with systemic administration is that if you exceed a certain level, a certain tissue or blood serum concentration, you enter the toxic spectrum, and human cells will be affected by the antibiotic as well,” explained Michiel Verhofstad, Professor of Trauma Surgery at Erasmus MC in Rotterdam, the Netherlands, who has been involved in the development of GEDAI-T as the chairperson of the AO Technical Commission’s Anti Infection Global Expert Committee. “However, if the dose is too low, bacteria will adapt to the new situation, and those less susceptible to the antibiotic will survive.”
Verhofstad sees clear advantages in local application: “When you apply antibiotics locally, you need much less to get the same concentration in the field of interest. And if you use the same amount in local application as you would in systemic treatment, you can achieve a much higher local concentration without the systemic side effects. If you have a high concentration, bacteria which are considered resistant to a certain antibiotic may become susceptible again.
However, the “how to” of applying antibiotics locally is an important question. Antibiotic powders added to the fracture site before the wound is closed will result in short-term high peaks and may spread throughout the body, eventually acting systemically. “What I, as a surgeon, would like to have is a dissolving carrier that contains the antibiotic and gradually releases it—and a gel would be my first choice,” said Verhofstad.
The formulation of GEDAI-T was developed by Matteo D’Este and the Biomedical Materials team at ARI, following specific requirements to its handling properties provided by surgeons from within the AO’s network, providing a highly versatile platform with the potential of bringing clinical benefit well beyond the prevention and treatment of FRI. It is one of several activities in the focus of ARI’s Infection Biology team under the lead of Fintan Moriarty, aimed at better understanding FRI to provide better treatments and improve clinical outcomes.
Antibiotic powders have been used off-label in the United States for a while now to treat and prevent FRI, but there is no gel on the market for this purpose, although a survey we did showed that it would be surgeons’ preferred manner of delivering antibiotics,” said Pamela Nylund, who is the Project Leader for the development of GEDAI-T at ARI. “The gel is very versatile and isn’t limited to fracture type or location.” Although the gel is currently being tested and approved with only one antibiotic—tobramycin—it could potentially carry and release other antibiotics and chemical substances in the future.
Over the following months, the gel will be applied in animal studies performed at ARI’s pre-clinical facilities to demonstrate its safety and efficacy. “We are looking at the effect on fracture healing, efficacy, and local and systemic toxicology,” said Andrea Montali, Manager of the AO’s Development Incubator. “We’re expecting to start the first clinical trials on humans in 2026 and are currently seeking industrial partners.” Following the initial development, which was conducted at ARI, the translational phase of the project is being funded and supported by AO’s innovation funding through its Development Incubator with the goal of compiling the necessary data, documentation and IP to make this an attractive business opportunity for a future industrial partner.
Although it will still take a few years for GEDAI-T to be fully approved, regulated, and market-ready, Verhofstad is confident it will eventually become a successful product: ”The gel has great potential. It is based on readily available and well-known raw materials with a long track record of use in the pharmaceutical and medical industries, and it dissolves gradually without leaving any footprint. The question is whether I would consider volunteering in a clinical study, and yes, I would.”
You might also be interested in:

