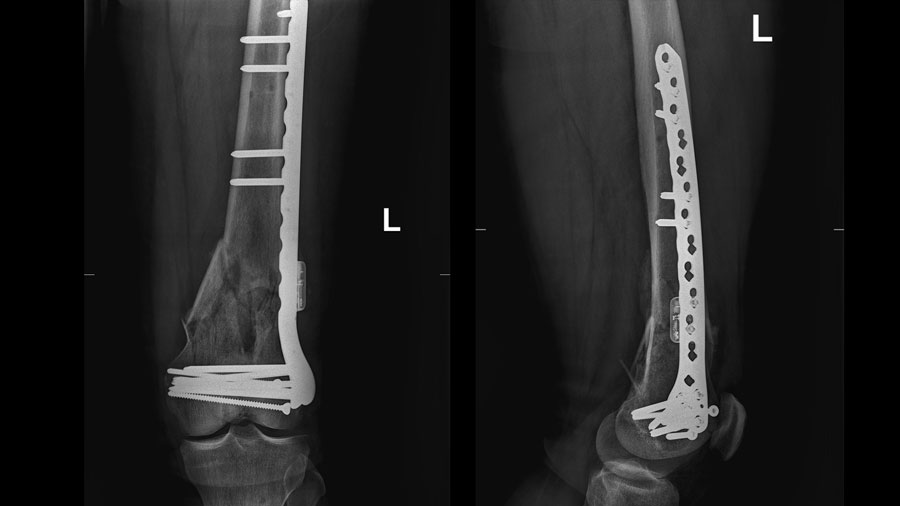
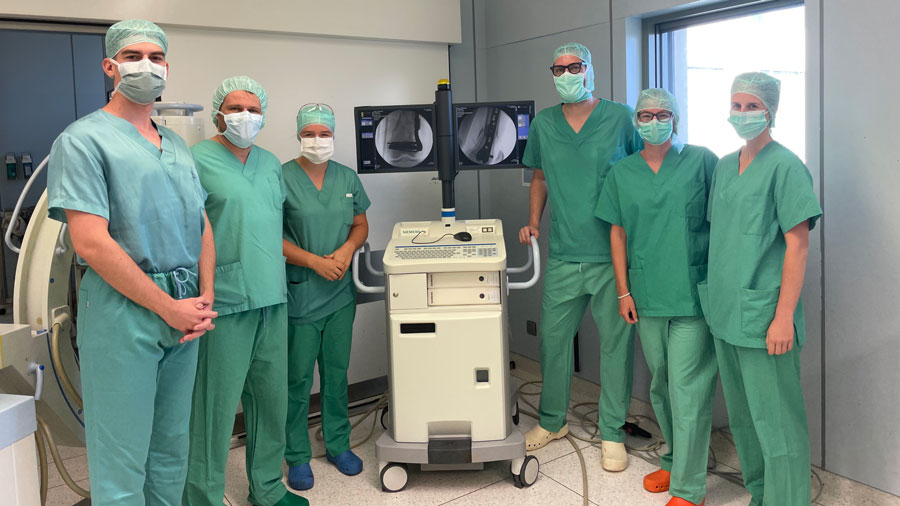
Patient convalescing following first-in-human AO Fracture Monitor application
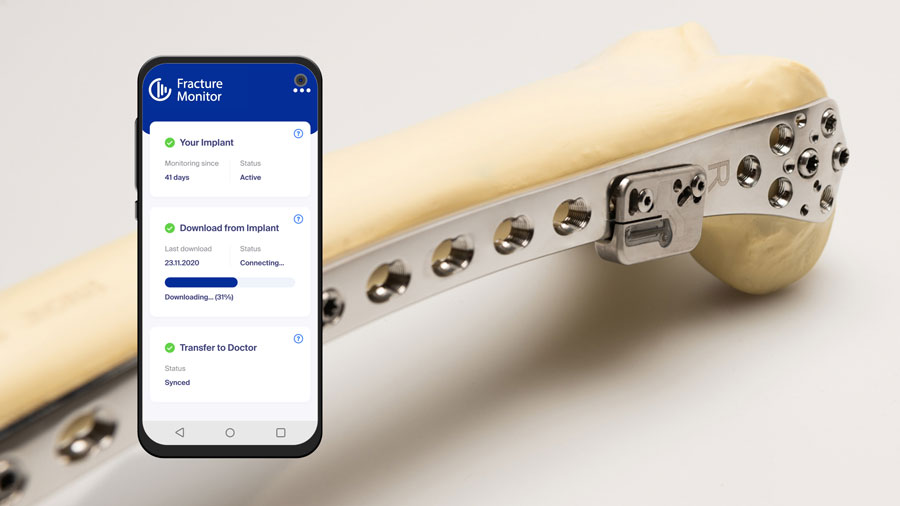
Sixty-five years after the AO was established to champion revolutionary internal fixation techniques that continue to achieve unprecedented results in fracture healing, its legacy of improving patient outcomes continues with the first-in-human application of the AO Fracture Monitor developed by the AO Research Institute Davos (ARI) with the AO’s innovation funding. In an important step toward regulatory approval, the safety of this implantable telemetric sensor system is now being validated in a multicenter clinical trial at four hospitals in Germany.
“He’s doing great and is very excited to be part of this study. All he has to do is check from time to time on the smartphone app that the data is transferred,” said Braun. “The patient is recovering on schedule and is happy with the treatment and study so far—but he would love to see the data, of course. My hope for this patient is that when he comes back six to eight weeks from now, he will be back to his preinjury condition.”
Many milestones
AO Research Institute Davos (ARI) Senior Project Leader Concept Development Manuela Ernst said the first-in-human application of the AO Fracture Monitor is just the latest of several milestones in the project’s history, but the most eagerly awaited one.
The system, which represents an innovative approach to individualizing patient rehabilitation, arose from talks between former ARI Focus Area Leader Concept Development Dr. Markus Windolf and AO founding father and longtime ARI Director Prof Stephan Perren. After many design iterations and fine-tuning, and eventually formal product development, a new kind of telemetric system was created, and the development team is happy to now have an active implantable device ready for clinical application. The current clinical study got underway in autumn this year with so far two patients enrolled. Over the next months, a total of 37 patients will be recruited at the participating hospitals BG Klinik Tübingen, Universitätsklinikum des Saarlandes, Universitätsklinikum Ulm, and Universitätsklinikum Münster.
The first-in-human application of the AO Fracture Monitor is an exciting milestone, Ernst said.
“It was an incredible experience to be able to observe the first surgery and actually see how the AO Fracture Monitor was implanted in a patient after so many years of development,” she recalled. The current study is expected to conclude in spring 2025 and provide the missing data for the subsequent conformity assessment by the notified body for market approval in Europe. “We anticipate a CE mark by the end of 2025 and availability for larger clinical studies by early 2026.”
‘A full-service resource’
Braun said AO innovation funding is critical in bringing new technologies to clinicians and promoting excellence in patient care and outcomes in trauma and musculoskeletal disorders.
“The AO Fracture Monitor is revolutionary in that it represents a step away from relying on traditional modalities like x-rays and toward a much more patient specific and accurate ways of monitoring bone healing,” he said. “The AO is helping make this project possible with not only funding but expertise at every step of development. To make a great idea a reality, you need strong partners, and the AO—with the AO Innovation Translation Center, including AO's innovation funding and Clinical Evidence teams, all the way to ARI—is truly a full-service resource.”
You might also be interested in:
- Discovering more about the AO Fracture Monitor
-
Learning more about the AO Research Institute Davos
-
Applying for the AO’s innovation funding
-
Reading about the first-in-human application of Biphasic Plate