AO Innovation Funding
Turn your musculoskeletal innovation into a clinical reality
Get customized funding, expert mentorship, and access to the biggest global network of orthopedic surgeons who will validate and use your innovation.
Apply by February 28, 2026
Proven impact: Our success stories
Watch: Discover how AO Innovation Funding accelerates breakthrough ideas.
Flexible funding: Customized amounts and timelines matched to your project milestones—from seed funding to clinical validation.
Expert network: Direct access to the biggest global network of orthopedic surgeons, researchers, and specialists who validate your solution and become early adopters.
De-risk your path: Navigate IP protection, regulatory approval, and clinical evidence with mentorship from leaders who have successfully brought musculoskeletal devices to market.
Industry credibility: AO backing signals quality to investors, hospital systems, and strategic partners, opening doors for commercialization.
We support innovations that address unmet clinical needs in musculoskeletal care:
- Hardware medical devices (implants, fixation systems, instruments)
- Digital health technologies (apps, AI, surgical planning software)
- Biologic solutions and/or combination devices
- Medical imaging solutions for AI applications
- Strategic initiatives advancing MSK treatment or surgeon education
Your innovation should:
- Demonstrate clear advantages over existing solutions
- Have IP protection potential (patent filed or patentable within five years)
- Show realistic path to market with strong business case
- Be suitable to scale and impact patient outcomes
How to apply
Simple 4-step process:
1. Submit application – Rolling submissions reviewed twice yearly (deadlines: February 28 & August 31)
2. Initial review (two months) – Expert panel evaluates diverse criteria depending on the project outcome, a medical device, or a strategic project for the AO.
3. Due diligence – If approved, we structure funding terms, milestones, and IP agreements
4. Project launch – Begin development with AO funding, mentorship, and network access

"A perfect place to look for innovation is where you see challenges."
Michael Schütz - Technology Transfer Board Chairperson
Meet the Governing teams
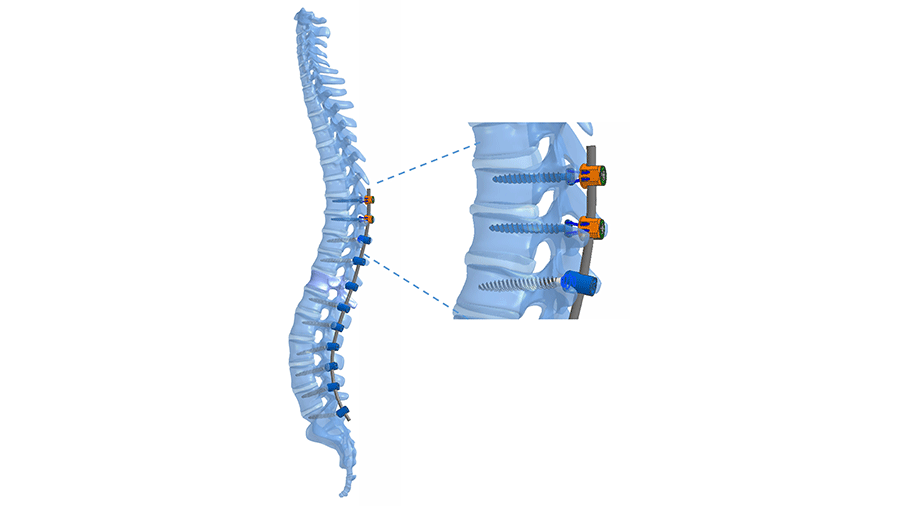
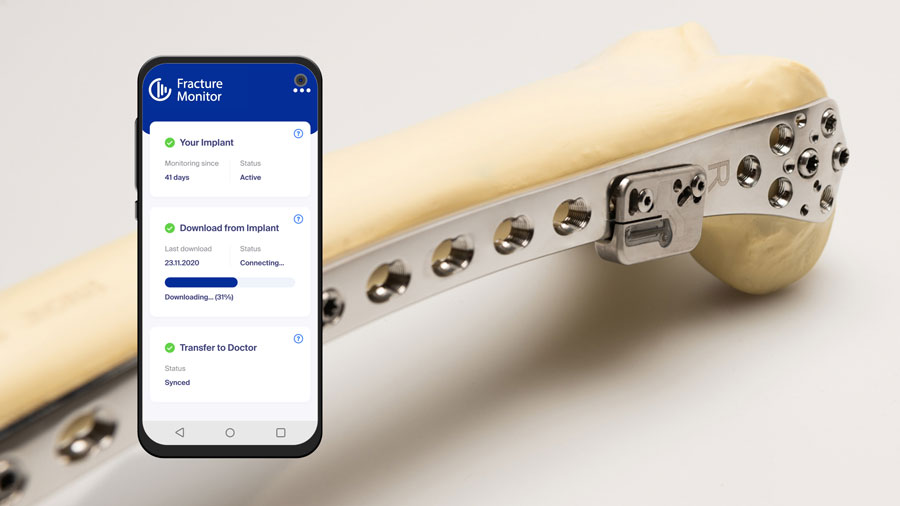
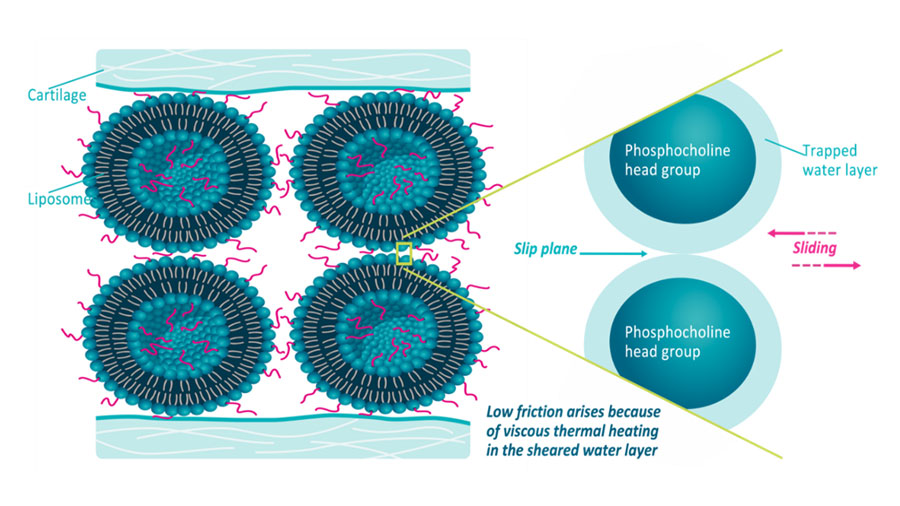
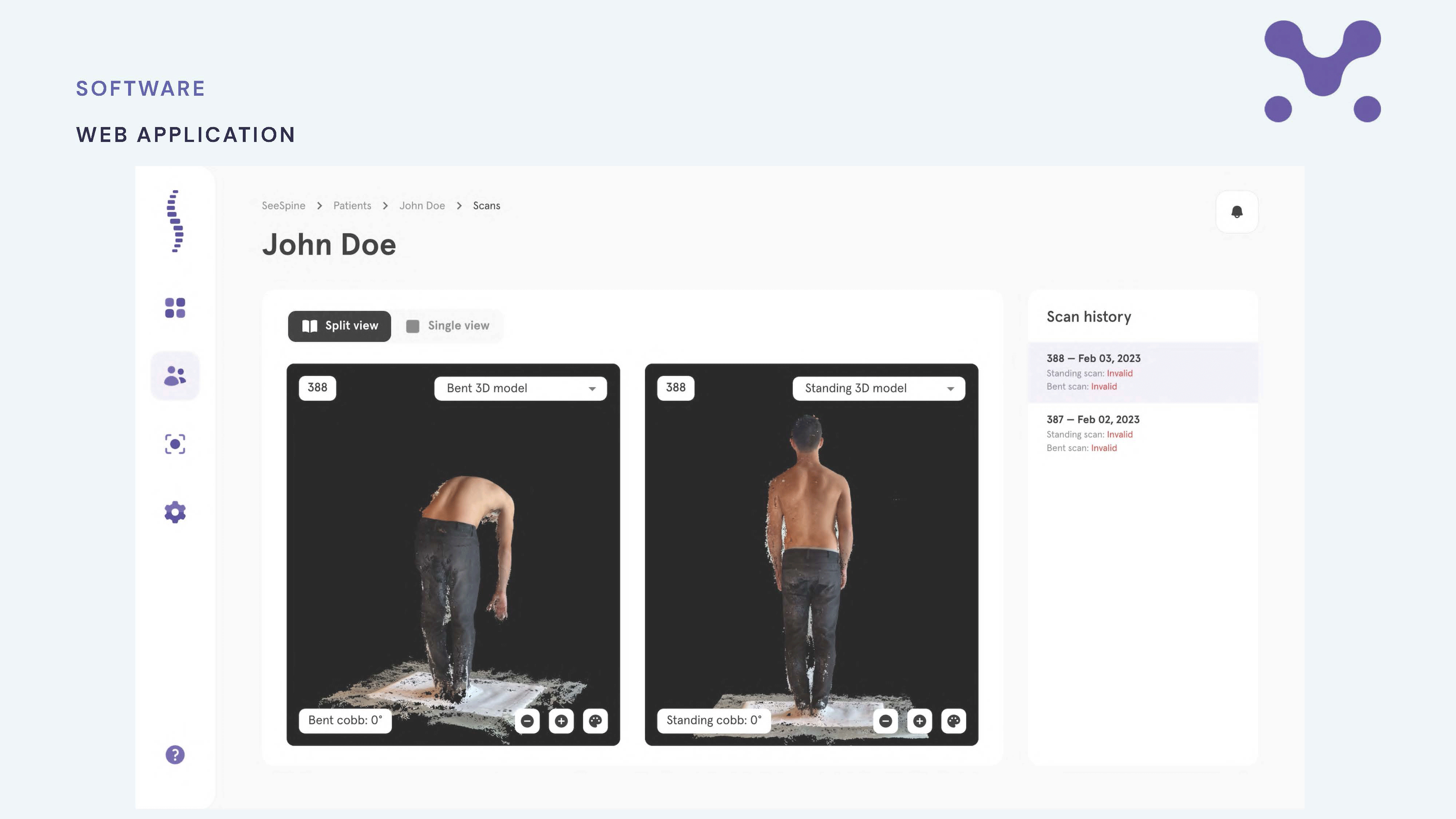
Latest innovation success stories
-
Innovation
-
Innovation
 December 11, 2019
December 11, 2019Let the AO help take your idea from paper, to patients
Apply to the AO Strategy Fund and AO Development Incubator today
-
Innovation
 December 01, 2019
December 01, 2019New AO calls for proposals: turn your ideas into reality
Apply to the AO Strategy Fund and AO Development Incubator today