Inside the healing process
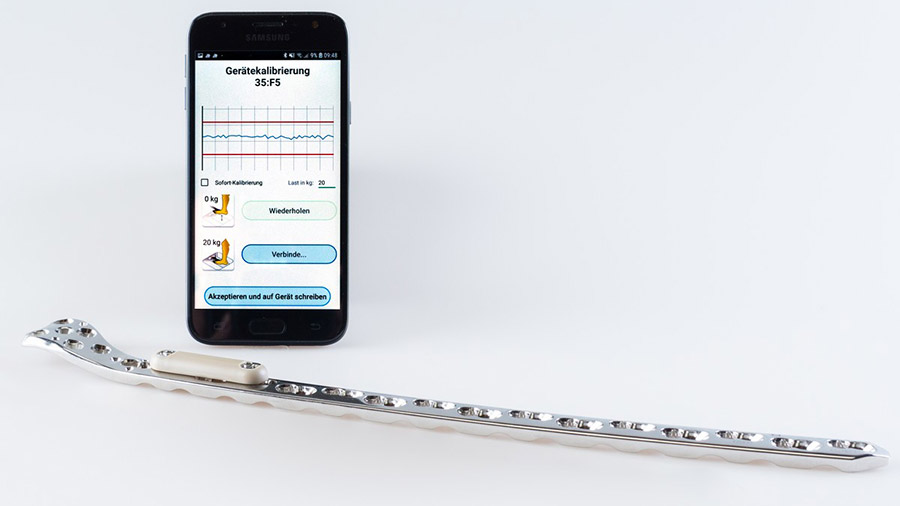
The concept involves an implantable data logger attached to a standard bone plate and a smartphone app that wirelessly connects with the data logger, downloading the information. A central web server collects and stores data from the patient’s smartphone for visualization, and the patient’s physician gains a high-value aid to their decision-making on further treatment.
“Measuring fracture healing through implant deformation is not a new idea, but continuously acquiring this information together with data on fracture activity is something new,” Windolf said. “A better understanding of how fractures heal and being able to objectively measure the healing process helps deliver better outcomes for patients.”
The AO Fracture Monitor measures the deflection of the stabilizing implant under physiological loading for up to eight months postoperatively and, at the same time, delivers patient mobility information. The project was initially funded by an AO Trauma Research Grant to develop the first prototype and measure fracture healing in the frame of animal experiments. An external fixation (ExFix) version of the device has been tested in clinical trials to obtain clinical proof of concept with strong support from the AO Technical Commission. Now, thanks to AO Development Incubator (AODI) support, the AO Fracture Monitor is moving towards patient trials in 2020. This AO Development Incubator support helps pave the way to the translation of this promising concept into clinical use.
“There is no device on the market that can objectively measure fracture healing. It can be considered a disruptive technology because x-rays are the current standard of care, and that sometimes can be difficult because it puts clinicians in the position of waiting as long as six months postoperatively to diagnose fracture healing complications—a period of patient suffering and lost productivity that could be significantly shortened with such technology,” Windolf said.
“Furthermore, we won’t need to fear reinjury of the bone because we will know when the patient is ready to fully bear weight on the fracture,” he added. “The earlier the patient can bear weight, the better, because that means the patient can go back to normal life as quickly as possible.”
Windolf said clinical trials to validate the implantable version of the AO Fracture Monitor are a key part of the support provided by the AO Development Incubator.
“Without AO Development Incubator support, there is no way we could finance such a project in the AO,” he said, explaining that the AO Development Incubator fosters novel concepts like the AO Fracture Monitor that support the AO Foundation’s mission of promoting excellence in patient care and outcomes in trauma and musculoskeletal disorders. That support ranges from financing to the know-how needed to build and execute project plans toward market introduction.
“The AO Development Incubator is a natural fit. It’s all about translating our ideas from research into clinical application. The AO Development Incubator is quite an important vehicle for us because it is willing to take on more potentially disruptive technologies.”
As with any innovative medical device, the AO Fracture Monitor could encounter regulatory and reimbursement hurdles, but Windolf is confident of the business case behind it.
“We are constantly receiving feedback from surgeons who indicate that the market is waiting for this solution,” he said.
In addition to Windolf, the AO Fracture Monitor project team at AO Research Institute Davos includes Manuela Ernst (Project Leader, Biomedical Development), Ronald Schwyn (Project Leader, Biomedical Development), Viktor Varjas (Project Leader, Biomedical Development), Dominic Gehweiler (Project Leader, Biomedical Development). Medical advisor is Prof Tim Pohlemann, a member of the AO Foundation Board.