Multiple crosslinked bio-inks for 3D microextrusion of tissue-like constructs and biodegradable thermoplastic elastomer for fuse deposition manufacturing
Background
Biofabrication is providing scientists and clinicians the ability to produce engineered tissues with desired shapes, chemical and biological gradients. Typical resolutions achieved with extrusion based bioprinting are at the macroscopic level. However, for capturing the fibrillar nature of the extracellular matrix (ECM), it is necessary to arrange ECM components at smaller scales, down to the sub-micron and the molecular level.
In this project, we introduce a (bio)ink containing hyaluronan (HA) as tyramine derivative (THA) and collagen (Col). Similarly, to other connective tissues, in this (bio)ink Col is present in fibrillar form and HA as viscoelastic space filler. THA was enzymatically crosslinked under mild conditions allowing simultaneous Col fibrillogenesis, thus achieving a homogeneous distribution of Col fibrils within the viscoelastic HA-based matrix. THA-Col composite displayed synergistic properties in terms of storage modulus and shear-thinning, translating into good printability.
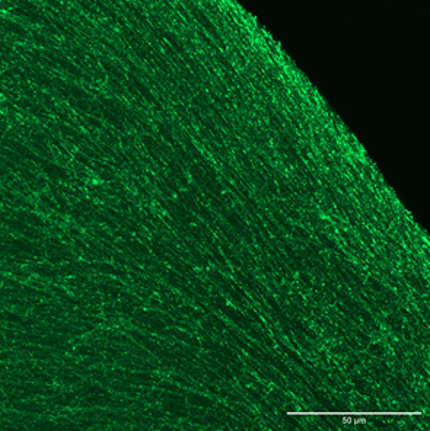
Shear-induced alignment of the Col fibrils along the printing direction was achieved and quantified via immunofluorescence and second harmonic generation (Figure). Cell-free and cell-laden constructs were printed and characterized, analyzing the influence of the controlled microscopic anisotropy on cell behavior and chondrogenic differentiation. THA-Col showed cell instructive properties modulating hMSC adhesion, morphology. Based on gene expression of cartilage/bone markers and matrix production, hMSCs embedded into the bioink displayed chondrogenic differentiation comparable or superior to standard pellet culture by means of proteoglycan production (Safranin O staining and proteoglycan quantification) as well as increase in cartilage related genes.
The possibility of printing matrix components with control over microscopic alignment brings
biofabrication one step closer to capturing the complexity of native tissues and the developed bioink can be integrated in the osteochondral implant of the OCD consortium.
-
Publication
Davidson MD, Ban E, Schoonen ACM, Lee MH, D'Este M, Shenoy VB, Burdick JA. Mechanochemical Adhesion and Plasticity in Multifiber Hydrogel Networks, Advanced Materials epub Dec 18 (2019) e:1905719.
-
Presentation
Petta D, Grijpma DW, Alini M, Eglin D, D'Este M. 3D Printing of a Hyaluronan Bioink With Double Gelation Mechanism for Independent Tuning of Shear-Thinning and Shape Fixation, Stem Cell Biology & Technology. Royan International Twin Congress. Reproductive Biomedicine & Stem Cells, Tehran.
Schwab A, Ambrosio L, Alini M, Eglin D, D'Este M. Anisotropic properties of a hyaluronic acid collagen biomaterial ink to control cellular behavior, TERMIS EU, Rhodos, 2019, p. 1422 / 883 (eCM coll).
Schwab A, Ambrosio L, Alini M, Eglin D, D'Este M. Hyaluronic acid collagen biomaterial ink with anisotropic properties to control cellular organization, SSB+RM Swiss Conference on Biomaterials and Regenerative Medicine eCM Periodical: Abstract Collections Muttenz, 2019, p. p19.
D'Este M, Beninatto R, Di Lucia A, Staubli F, Schwab A, Galesso D, Pavan M, editors. Printability and cytocompatibility of a photo-initiator-free bioink based on coumarin-modified hyaluronic acid and gelatin. 2021 TERMIS world congress digital (poster)
Palladino S, Schwab A, D'Este M, Copes F, Candiani G, Mantovani D. Innovative bioink from collagen and hyaluronic acid with tunable rheological and biological properties for cardiovascular 3D bioprinting. 2021 TERMIS world congress digital (oral)
Schwab A, Staubli F, Eglin D, D'Este M, editors. Effect of biomaterial composition on MSC chondrogenesis embedded in a hyaluronan composite containing collagen fibrils. 2021 TERMIS world congress digital (oral)
Schwab A, Wesdorp MA, Loebel C, Levato R, Burdick JA, Malda J, Stoddart M, van Osch GJVM, Eglin D, D'Este M. Ex vivo migration of chondrocytes - cell invasion into acellular biomaterials filled in a cartilage ring model is dependent on biomaterial composition. 2021 TERMIS world congress digital (oral)
-
Partner
Malda J (Prof) and Levato R (PhD), The University Medical Center Utrecht, the Netherlands
Bastiaansen-Jenniskens YM (PhD), Narcisi R (PhD) and van Osch G (Prof), The University Medical Center Rotterdam, the Netherlands
Ho K (MD, PhD), Qin L (Prof, MD), Chinese University of Hong-Kong, Hong-Kong
Burdick J (PhD), Mauck R (Prof), the University of Pennsylvania, USA