Inducing bone regeneration through immuno-modulation of biomaterials (RAIMBO)
Background
Bone repair in the craniomaxillofacial region is still a clinical challenge with a major impact on patients. For large defects after trauma or tumor resection, bone autografts are generally adopted as the clinical standard. However, this solution is far from ideal as donor site morbidity and the limited amount of material available impose limitations. Bone graft substitutes from natural or synthetic biomaterials would be a valid alternative, although their efficacy is hampered since they do not have the intrinsic healing properties of viable autologous bone. Additionally, they may still trigger deleterious immune responses, such as fibrous encapsulation, resulting in impaired new bone formation and possibly even leading to implant rejection.
Adaptive and innate immune cells such as T cells, macrophages and neutrophils play a central role in modulating the immune responses to biomaterials used in the fabrication of implantable devices. While some general principles governing the immune response to implanted biomaterials topography and chemistry have been investigated, the consequences of interactions of 3D printed constructs with the host immune system remains mostly unknown. The systematic knowledge of how variations in shape, topochemistry and composition of 3D printed constructs interacts with the host immune system opens the possibility of modulating the immune response to biomaterials, and ultimately to improve bone healing. The overall goal of this project is to investigate the effect of specific material properties on immune cells such as neutrophils or macrophages. This will contribute to the design of biomaterials inducing a “healthy” inflammation in target clinical problems such as osteointegration; infection; fibrous encapsulation; osteolysis; implant rejection; new bone formation.
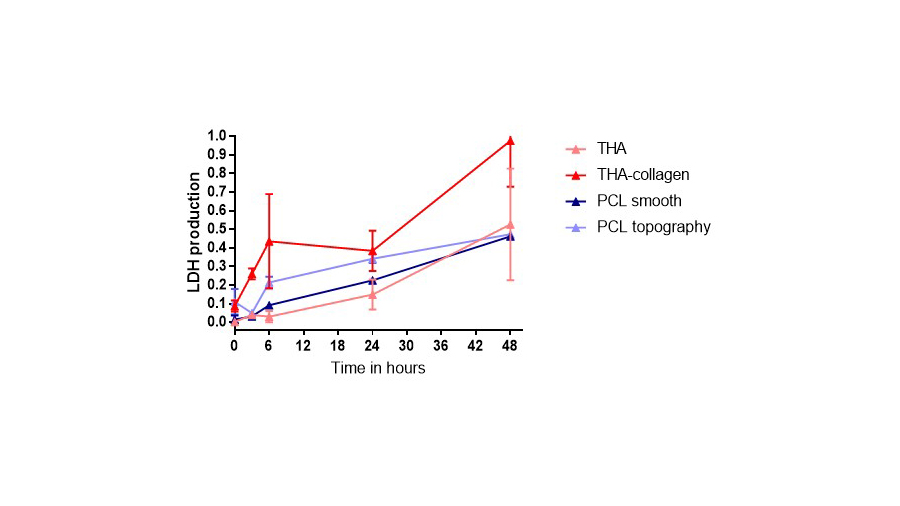
Experiments were performed to assess cell attachment, survival, LDH and reactive oxygen species production using a neutrophil-like cell line (PLB-985). LDH increased for collagen-THA in comparison to THA, and for PCL with surface topography rather than smooth (Figure). For validating the results, primary neutrophils from peripheral blood will be employed in the incoming experiments. For obtaining human blood, we applied for ethical approval at Swiss Ethics.
-
PublicationWesdorp MA, Schwab A, Bektas EI, Narcisi R, Eglin D, Stoddart MJ, Van Osch GJVM, D'Este M. A culture model to analyze the acute biomaterial-dependent reaction of human primary neutrophils in vitro. Bioact Mater. 2023;20:627-37. https://doi.org/10.1016/j.bioactmat.2022.05.036