Identifying novel therapeutic targets for articular cartilage repair
Background
Novel therapies for cartilage regeneration have had limited success. Chondrogenic differentiation of mesenchymal stem cells (MSCs) under load is different to that observed during classical static culture conditions. This is highly clinically relevant, considering that patients receive weight-bearing rehabilitation therapy following cartilage repair. Additionally, as most in vitro cartilage repair studies are performed under static conditions, the lack of mechanical stimulation may explain why it has been challenging to reproduce promising in vitro results in vivo. Marrow stimulation techniques, such as microfracture, are the most commonly used clinical approach for cartilage repair with unpredictable results. Using a unique in vivo kinematic join simulating bioreactor, we have previously shown that while complex multiaxial load induces hMSC chondrogenesis, it also induces the expression of a number of soluble molecules not typically found under static culture conditions. This identified novel mechanically induced targets, such as nitric oxide (NO), that are potentially clinically relevant. Within this project we aim to better understand the role of mechanical load on the molecules induced during human MSC chondrogenesis vs standard conditions (static and with TGF-beta). We will identify new potential treatment targets, while investigating the biological function of nitric oxide.
Goal
This project aims to establish the functional modulation of non-cartilage cell types by mechanically stimulated MSC secretome, thus providing valuable further insight into the pathology of joint destruction.
Results
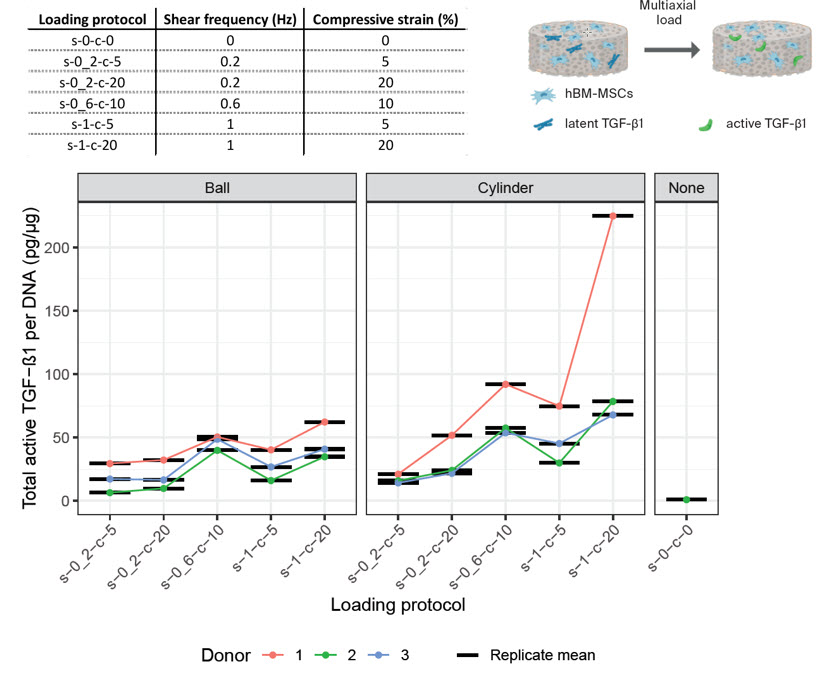
Using a design of experiments (DoE) approach, we have established optimal loading conditions to a) increase TGFβ production and b) increase the mechanical activation of latent TGFβ protein. Interestingly the protocol optimal for expression is not the same as that optimal for activation. This suggests that rehabilitation protocols may need to increase in complexity to improve cellular differentiation.
Fund:
Swiss National Funds (nr 31003A_179438 / 1), Funding: CHF 417'720, Period: 08/2018-07/2022
-
Publication
Stoddart MJ, Hofmann S, Holnthoner W. Editorial: MSC Signaling in Regenerative Medicine. Front Bioeng Biotechnol. 2020;8:1276.
Monaco G, Ladner YD, El Haj AJ, Forsyth NR, Alini M, Stoddart MJ. Mesenchymal stromal cell differentiation for generating cartilage and bone-like tissues in vitro.
Cells. 2021;10(8):2165. https://doi.org/10.3390/cells10082165Ladner YD, Armiento AR, Kubosch EJ, Snedeker JG, Stoddart MJ. Optimization of loading protocols for tissue engineering experiments. Sci Rep. 2022;12(1):5094. https://doi.org/10.1038/s41598-022-08849-y
Huber S, Ladner Y, Stoddart MJ, Leunig M, Ferguson SJ. The acetabular labrum tissue shows unique transcriptome signatures compared to cartilage and responds to combined cyclic compression and surface shearing. Gene. 2023;856:147140. https://doi.org/10.1016/j.gene.2022.147140
Ladner YD, Alini M, Armiento AR. The Dimethylmethylene Blue Assay (DMMB) for the Quantification of Sulfated Glycosaminoglycans. Methods Mol Biol. 2023;2598:115-21. https://www.doi.org/10.1007/978-1-0716-2839-3_9
Stoddart MJ, Della Bella E, Armiento AR. Cartilage Tissue Engineering: An Introduction. Methods Mol Biol. 2023;2598:1-7. https://www.doi.org/10.1007/978-1-0716-2839-3_1
Ladner YD, Kasper H, Armiento AR, Stoddart MJ. A multi-well bioreactor for cartilage tissue engineering experiments. iScience. 2023;26(7):107092 https://doi.org/10.1016/j.isci.2023.107092
-
Presentation
Ladner Y, Armiento AR, Snedeker JG, Stoddart MJ. Full factorial design of experiment elucidates interaction of mechanical parameters driving in vitro cartilage formation in a bioreactor. 2020 GR forscht virtual (oral).
Ladner YD, Armiento AR, Snedeker JG, Stoddart MJ. Controlled factorial design of experiment to optimize mechano-induced chondrogenesis of human MSCS. 2020 EORS virtual (oral)
Stoddart M. Regenerative rehabilitation: from proteins to patients. 2021 TERMIS world congress digital (oral)
Stoddart M. Cell therapy for cartilage repair-modelling - Increasing complexity of cartilage models. 2022 ICRS (oral)
Ladner YD, Armiento AR, Stoddart MJ. A multi-well bioreactor for cartilage tissue engineering. 2022 SSB+RM (poster)
Ladner Y, Armiento A, Stoddart M. A multi-well bioreactor for cartilage tissue engineering. 2022 TERMIS EU (poster)
Ladner Y, Armiento A, Stoddart M. Mechano-induced chondrogenesis of human MSCs in a biomaterial: A factorial design of experiment approach. 2022 TERMIS EU (poster)
Stoddart M. Reproducing kinematic load in vitro for chondrogenesis studies. 2022 TERMIS AP (oral)
Stoddart M. Regulating MSC fate under complex mechanical load. 2023 TERMIS-EU (oral)
-
Partner
Snedeker Jess G (Prof, PhD), ETH Zürich, Switzerland