3D printing of cellularized tissue engineered constructs. Bioinks and methods toward clinical translation (Bioink)
Background
Patient specific implants based on additive manufacturing principles hold great promise in CMF applications, where anatomical fidelity is paramount. As the imaging to printing workflows improve, one of the major remaining hurdles is the development of bioinks that are osteoinductive, while at the same time having a realistic path through regulatory approval.
By avoiding complex material developments and following a "less is more" approach, we believe a novel material with clinical approval can be obtained more rapidly. This project investigated the printability of three clinically approved natural materials based on hyaluronic acid, fibrin and collagen. Further addition of Polyphosphate nanoparticles and dexamethasone releasing microparticles was investigated, with osteogenic differentiation the measured outcome. The potential to improve the gels osteoinductive properties by the addition of simple osteoinductive molecules that are already clinically approved would have a less challenging approval process.
Goal
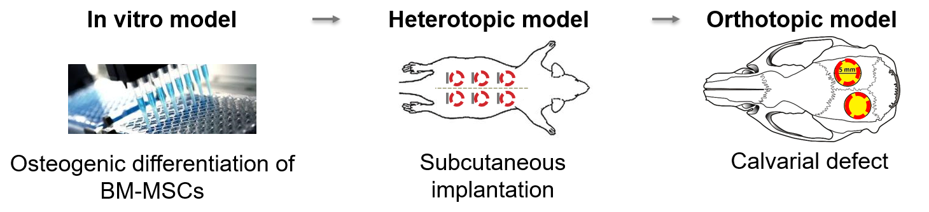
To develop 3D printing of clinically relevant biopolymer hydrogel products, namely collagen type I, fibrin glue and hyaluronan for the manufacturing of cellular 3D osteogenic constructs.
Results
We have investigated several materials, with or without biological enhancers, for their bone forming capabilities both in vitro and in vivo. Surprisingly, the results of these assays often do not correlate, suggesting that current material testing algorithms are sub-optimal. Osteogenic differentiation was generally poor in all the materials tested, while polyphosphate addition appears to increase osteogenic differentiation in vitro. This project highlights the need for more suitable in vitro testing models for osteogenesis, in order to more accurately screen novel materials in the early stages of the development process.
-
Publications
Hatt LP, Thompson K, Müller WEG, Stoddart MJ, Armiento AR. Calcium Polyphosphate Nanoparticles Act as an Effective Inorganic Phosphate Source during Osteogenic Differentiation of Human Mesenchymal Stem Cells. Int J Mol Sci. 2019 Nov 18;20(22). pii: E5801. doi: 10.3390/ijms20225801
Hatt LP, Armiento AR, Mys K, Thompson K, Hildebrand M, Nehrbass D, Müller WEG, Zeiter S, Eglin D, Stoddart MJ. Standard in vitro evaluations of engineered bone substitutes are not sufficient to predict in vivo preclinical model outcomes. Acta Biomater. 2022;epub Aug 18. https://www.doi.org/10.1016/j.actbio.2022.08.021
-
Presentations
Hatt LP, Thompson K, Mülller WEG, Stoddart MJ, Armiento AR. Calcium polyphosphate-nanoparticles act as an effective inorganic phosphate source during the in vitro osteogenic differentiation of human bone marrow-derived MSCs. 2019 SBMS (oral)
-
Partners
Werner E G Müller (Prof) Institute for Physiological Chemistry, University Medical Center of the Johannes Gutenberg University, Mainz, Germany