Background
Information on healing progression and load-bearing characteristics in fracture patients is only barely tapped due to the inaccessibility of a confined biological region and the limited value of radiographic methods. A novel approach to continuously measure both fracture healing and patient activity has been recently developed at ARI. The system comprises an implantable data logger for autonomous collection of relevant parameters to access fracture healing. Wireless synchronization of the acquired healing data via patient's mobile phone allows for remote monitoring by the treating physician. Proof of concept is obtained from preclinical experiments and from first clinical data collection with prototype devices on external fixation (Project SmartFix).
Goal
The AO Fracture Monitor shall be further developed into a commercially applicable system for large fragment bridge plating. The implantable device plus the accompanying software shall be developed and tested according to the regulatory requirements and undergo clinical evaluation thereafter.
Results
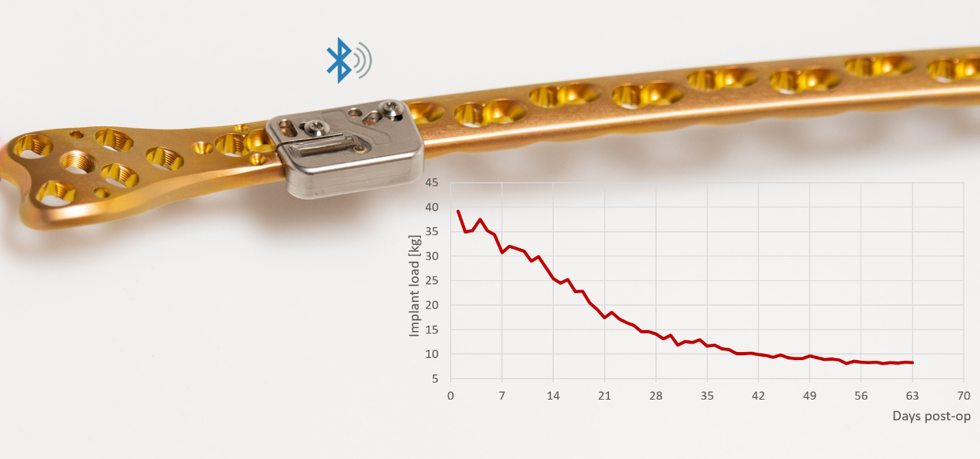
A comprehensive refinement of the previous implant prototype has been finished in 2019, resulting in an implantable device that integrates well with the locking plate with minimal elevation over it. It is hermetically encapsulated in a metal enclosure and provides continuous healing data collection and processing approximately 6 months long. A cloud solution is currently being set up to store all patient data and to display the information in an intuitive way to the treating physicians.
The first 10 animals were operated in 2019 with the new implantable data logger attached to a 10-hole LCP bridging a tibial defect. The defect size ranges from 0.6 to 10 mm to generate different healing times. Up to now, all devices have operated flawlessly, delivering continuous healing data. The collected data demonstrates the capability of the fracture monitor to differentiate different stages of healing at an early phase. Monitoring will continue until the end of the implant's lifetime. Final evaluation will be performed 9 months post operation.
In parallel to the preclinical project part, the detailed design of the AO Fracture Monitor has been pushed forward so that it can be transferred for production process development and subsequent verification and validation, including the first application in a clinical trial.
-
Publication
Comtesse S. Prediction of Interfragmentary Movement, Loading and Implant Strain based on Finite Element Modelling and Strain Measurements with the AO Fracture Monitor, MSc ETHZ Biomedical Engineering, 2019 (Ferguson SJ, Ernst M, Mischler D) (Thesis)
Windolf M. Device for measuring, processing and transmitting implant parameters. CH01335/19 (Patent)
Ernst M, Richards RG, Windolf M. Smart implants in fracture care – only buzzword or real opportunity? Injury. 2020;epub Sept 17.
Windolf M, Varjas V, Gehweiler D, Schwyn R, Arens D, Constant C, Zeiter S, Richards RG, Ernst M. Continuous implant load monitoring to assess bone healing status - evidence from animal testing. Medicina. 2022;58(7):858
-
Presentation
Windolf M. Lessons in overcoming challenges—from the team that developed the AO Fracture Monitor, DKOU 2019, Berlin, Germany (invited oral).
Ernst M. AO Fracture Monitor and the necessity of translational medical education, sitem-insel School: Bringing Innovation to the Patient, 2019, Bern (oral).
Gueorguiev B. From Wearables to Smart Implants – AO Fracture Monitor to Assess Bone Healing, ICORS 2019, Montreal, Canada (oral).
Richards RG, Ernst M. Digitalized aftercare in orthopaedics. DDF 2021 virtual (oral).
-
Partner
Pohlemann T (Prof), University Clinic Saarland, Homburg, Germany