Host-pathogen interaction
-
Staphylococcus aureus colonization of canaliculi during bone infection
Discovery of a previously unknown mechanism whereby Staphylococcus aureus deforms into rod-shaped bacteria ultilizing osteocytic-canalicular networks to propagate deep into live bone tissue.
Karen Bentley, University of Rochester, United States
This project has led to the discovery of something new about the behavior of Staphylococcus aureus (S. aureus) and why it may resist antibiotic treatment and recur in patients who have had a hip or joint implant.
Transmission electron microscopy (TEM) was utilized to examine the bones of mice with implant-associated S. aureus chronic osteomyelitis. While examining thin (70 nm) bone sections at high magnification, it was observed that some bacteria were able to change shape and squeeze into submicron spaces called canaliculi. The study documented how round bacteria become rod-shaped to accommodate the canaliculi submicron diameter space. These bacteria propagate deep into bone, infecting osteocytes. Because bone is an immune-privileged tissue, bacteria are protected from treatment with traditional antibiotics delivered through blood vessels.
This finding is significant, as S. aureus has never been described as being able to deform. It has always been described as round, 1 µm in diameter, and growing in clusters like grapes on a vine. Soon after documenting this bizarre shape-shifting behavior in a mouse model, the same bacterial phenomenon was discovered to occur in human bone, confirming the clinical significance of the discovery.
These initial findings explain, among other things, why a S. aureus infection in humans may return despite weeks or months of antibiotic treatments and bone debridement when replacing an infected implant. It also explains why infections can recur, sometime years—even decades—later, after going undetected in the patient.
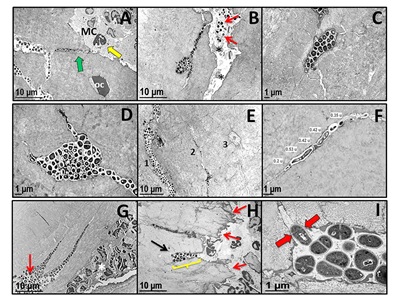
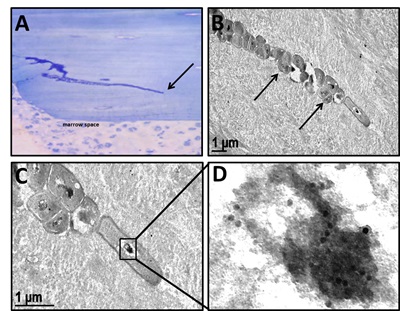
Figure 1. TEM evidence of submicron-elongated S. aureus in the osteocytic lacunar-canaliculi network of infected live bone tissue. (A-F, H, I) Long bones from mice (n = 5) infected with a UAMS-1-contaminated tibial pin or (G) a USA300-infected femoral osteotomy were harvested on day 14 post infection for TEM. (A) Low magnification TEM image (x 4,000) of UAMS-1 invasion of live bone tissue (note osteocyte OC) in a canaliculus (green arrow) communicating with the marrow cavity (MC). Also note the proximal neutrophils (yellow arrow) within the marrow. (B) Low magnification TEM image (x 4,000) of UAMS-1 invasion of an osteocytic lacunar canaliculus adjacent to a channel infected with S aureus (arrows) containing necrotic cells (*). Higher magnification TEM images (C: x 8,000; D: x 10,000) of UAMS-1 colonization of osteocytic lacunae. (E) Low magnification TEM image of three parallel canaliculi in various states of colonization (1: severely infected; 2: moderately infected, and 3: uninfected) by the invading UAMS-1 within the live cortical bone (x 3,500). (F) Higher magnification TEM image measuring submicron-elongated UAMS-1 (x 15,000). (G) Similar bacterial invasion of canaliculi adjacent to the osteotomy (red arrow) and neutrophils in the marrow cavity (*) were observed in USA300-infected femurs (x 4,000) but not in long bones that received sterile implants (data not shown). (H) Low magnification TEM image (x 4,000) documenting cortical bone damage adjacent to the infected tibia pin (red arrows) and a cavity filled with UAMS-1 (yellow bracket) that leads to a canaliculus (black arrow). (I) High magnification TEM of the infected cavity in H demonstrating mitotic S. aureus in the live cortical bone (x 25,000). Note that only the bacterium entering the canaliculus has an asymmetric septal plane (red arrows), which is aligned perpendicularly with the canaliculus orifice, perhaps to anchor and propel the emerging daughter cell into the submicron channel in the cortical bone during binary fission.Figure 2. Immunoelectron microscopy (IEM) evidence of proliferating submicron-elongated S. aureus at the leading edge of canalicular invasion. Mice (n = 5) received a femoral osteotomy which was infected with S. aureus (UAMS-1), fed BrdU in their drinking water every day of the infection and their femurs were harvested on day 14 post-operation for IEM. (A) Light microscopy image (x 4) of the toluidine-blue-stained cortical bone section containing a 0.125 mm microcrack colonized with S. aureus up to the leading edge (arrow), which was interrogated by IEM. (B) Low (x 15,000), (C) high (x 40,000), and (D) ultrahigh (enlarged from 150,000 original) magnification IEM images of BrdU-positive S. aureus at the leading edge of the colonized canaliculus. (C) Note the rod-shaped bacterium with a diameter of 0.36 µm at the leading edge of bacterial infiltration and the immuno-gold labeled chromosome (12 nm black dots in D) confirming BrdU incorporation. IEM on S. aureus-infected femurs from mice that were not fed BrdU were all negative for immuno-gold labeling (data not shown).
-
Adaptive upregulation of clumping factor A (clfA) by S. aureus is critical in mediating increased osteomyelitis risk in obese/type 2 diabetic hosts
Defining host and bacterial factors that contribute to higher risk of implant-associated Staphylococcus aureus (S. aureus) infections in obesity-associated type 2 diabetes and novel interventions for prevention and treatment.
Robert Mooney and Steven Gill, University of Rochester, United States
Obesity and type 2 diabetes (T2D) are among the highest risk factors for orthopedic infection and poor outcomes following total joint arthroplasty (Cram et al, 2012; Del Pozo et al, 2009). Despite this, little is known about the mechanisms driving increased infection susceptibility and increased risk of complications in obesity/T2D. To address this knowledge gap, we have developed a mouse model of orthopedic implant-associated infection in the background of high-fat (HF) diet-induced obesity/T2D that recapitulates the more severe pathology seen in S. aureus osteomyelitis in obesity/T2D conditions. Using this model, we demonstrated that obesity/T2D is associated with worse S. aureus osteomyelitis, reduced anti-S. aureus immunoglobulin G (IgG) levels, and evidence of B cell defects (Farnsworth et al, 2015; 2018). We have also shown a more reactive bone formation and more osteolysis in obese/T2D mice (Farnsworth et al, 2017a). While these observations provide new insights into the contribution of the host to the more severe infection, S. aureus is well known to adapt to various environments to potentiate survival and infection. We asked whether S. aureus infection severity in obesity/T2D is at least in part due to adaptive changes in this organism in response to the unique environment of the obese/T2D host.
S. aureus adapts to the unique environment of the obese/T2D host
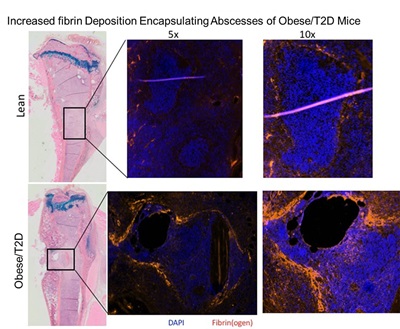
Using our model of orthopedic-implant-associated infection in the obese/T2D mice, expression of four genes coding for S aureus surface proteins was found to be markedly elevated in obesity/T2D. All code for cell surface fibrinogen-binding proteins: clfA (34-fold), bbp (42-fold), sdrC (48-fold), and clfB (19-fold) (Farnsworth et al, 2017b). Obesity and diabetes are known to be hypercoagulable states (Kannel et al, 1990; Yarmolinsky et al, 2016). Increases in fibrinogen and plasminogen activator inhibitor-1 (PAI-1) and activation of several clotting factors are well documented. It has been argued that these changes contribute to the increased risk of cardiovascular events and stroke in this population. Thus, it is very intriguing that S. aureus expresses markedly higher levels of fibrinogen-binding proteins in the obese/T2D host. Importantly, our most recent data show that our mouse model replicates the hypercoagulable state of obese/T2D patients. Additionally, S. aureus abscesses in the medullary canal following implant infection are encapsulated by a more prominent fibrin structure in the obese/T2D mice (Figure).
Figure: Increased fibrin deposition surrounds S. aureus abscesses in infected tibias from obese/T2D mice. Lean and obese/T2D mice were subjected to a S. aureus-coated pin placement through the tibia. At day 14, mice were sacrificed and histological sections prepared and stained with anti-fibrin(ogen) and a fluorescent-labelled secondary antibody. Sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Low-power view of stained section is presented for reference. Magnification represents power of the objective lens.
Elevated circulating fibrinogen in the obese/T2D state may synergize with the upregulation of clfA to enhance S. aureus virulence and colonization by providing the ligand for clfA (and the other fibrin(ogen) binding proteins). These potentially impactful new insights into S. aureus adaptation and colonization in obesity/T2D are the basis for our current work. The hypothesis being tested is that obesity/T2D and its hypercoagulable state promote more severe implant-associated S. aureus colonization and abscess formation by promoting fibrin(ogen)-specific adaptive gene expression changes, specifically a marked induction of clfA.
A critical step is to determine the role of ClfA in S. aureus adaptation in obese/T2D mice
Remarkably, the more severe infection associated with obesity/T2D was totally blunted in mice infected with a clfA null S. aureus mutant. Fewer bacteria were isolated from the infected bone, fewer abscesses were observed in the medullary canal of the infected tibia, and less osteolysis was observed. In fact, levels of these endpoints were comparable to those of lean mice. In other words, the impact of obesity/T2D was eliminated. These data argue that increased expression of the fibrin(ogen) binding protein ClfA by S. aureus is critical to the increased virulence of this bacteria in the obese/T2D state.
To begin dissecting the factors that mediate the adaptive changes in S. aureus to the host environment, an in vitro experimental system is now being utilized. A modification of the classic coagulase assay is used in which S. aureus is incubated with citrated plasma from diabetic and non-diabetic patients (as determined by HbA1c levels). The rationale for using human plasma is the appreciation that adaptation to human host factors is most critical to this investigation. In the presence of S. aureus, citrated plasma obtained from diabetic patients yielded fibrin deposition that exceeded that of non-diabetics by a factor of 2. Comparable results were obtained with plasma from lean vs obese/T2D mice. These results confirm a hypercoagulable state in the obese/diabetic host that can be exploited by S. aureus. In further refinement of this in vitro approach, recent studies indicate that S. aureus up-regulates clfA expression in the presence of plasma from diabetic patients. Additionally, fibrinogen alone can replicate this selective upregulation of clfA. These exciting results begin to define a cause and effect relationship between S. aureus virulence changes through adaptive gene expression and the hypercoagulable state of obesity/T2D. Future studies will focus on the specific mechanism mediating the above effects and potential efficacy of passive anti-ClfA antibody therapy in obesity/T2D to ameliorate infection severity.
Additional References
Cram P, Lu X, Kates SL, Singh JA, Li Y, and Wolf BR (2012) Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991-2010. JAMA 308: 1227-1236.
Del Pozo JL, Patel R (2009) Clinical practice. Infection associated with prosthetic joints. N Engl J Med 361: 787-794.Yarmolinsky J, Bordin Barbieri N, Weinmann T, Ziegelmann PK, Duncan BB, Ines Schmidt M (2016) Plasminogen activator inhibitor-1 and type 2 diabetes: a systematic review and meta-analysis of observational studies. Scientific reports 6: 17714.
Kannel WB, D'Agostino RB, Wilson PW, Belanger AJ, Gagnon DR (1990) Diabetes, fibrinogen, and risk of cardiovascular disease: the Framingham experience. Am Heart J 120: 672-676.
The pillars of the CPP Bone Infection
Host-pathogen interaction
Learn about how S. aureus adapts to the environment of an implant-associated infection.
Diagnosis
Read about the development of new tools to assess implant-associated infections.
Prevention
Explore how the CPP Bone Infection is running its surgical site prevention program.
Publications
Check the publications of the CPP Bone Infection