Bone Loss in Tibial Fractures
BY MITCHELL BERNSTEIN AND MARK LEE
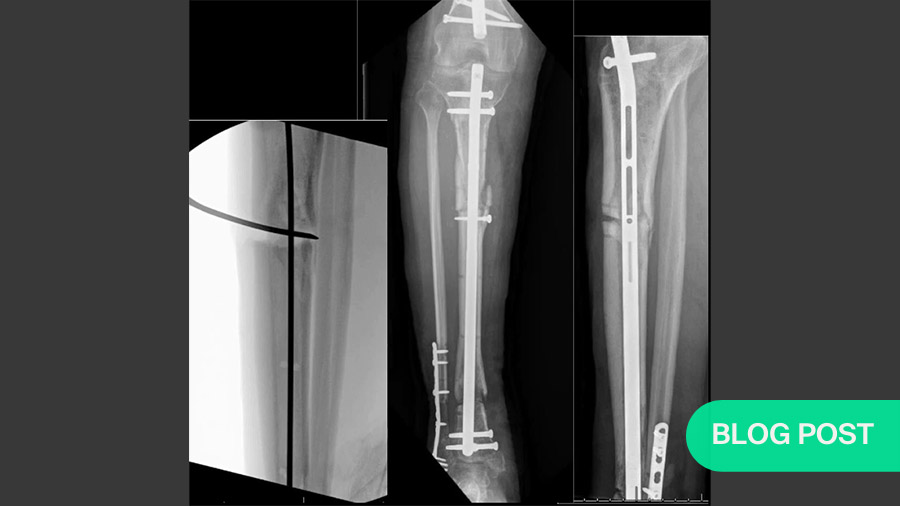
Meta- and diaphyseal tibial bone loss is quite rare in orthopedic trauma, making it difficult to set up large-scale studies. Its most common causes are trauma, infection or the excision of tumors. Complications—especially infection—can lead to long-term loss of function or amputation. And while various treatments are available, no consensus yet exists regarding which are most effective.
The current top intervention choices
The most common treatments for large critical sized bone defects are the Ilizarov Method (distraction osteogenesis), cancellous bone grafting, and vascular fibular autograft. Limitations for fibular autograft transfer include having the services of a specialized microsurgeon and carries the risk of stress fractures in both graft and donor sites. And while Ilizarov’s technique is extremely reliable it can be cumbersome for the patient.
A rising star
One increasingly popular alternative to these long-standing solutions is Masquelet’s induced membrane technique. This blog summarizes and supplements an excellent and highly-engaging AO webinar in which two orthopedic trauma surgeons—Mitchell Bernstein in Montreal and Mark Lee in Sacramento—discuss their experiences and impressions regarding the induced membrane technique. Along the way, Lee presents five case studies, each demonstrating a different treatment option, along with innovative combinations of therapeutic elements. Contrasting examples are included using the Ilizarov Method, highlighting strategies to integrate internal fixation to allow for an improved patient experience.
Learning Objectives
- Describe the steps and challenges of Masquelet’s induced membrane technique for the distal tibia.
- Avoid complications with the induced membrane technique for the distal tibia.
- Describe advanced techniques for bone regeneration for bone loss in the distal tibia.
- Describe unique approaches to bone defect reconstruction for challenging bone defect cases.
I. What is Masquelet’s induced membrane technique and how does it work?
Masquelet’s induced membrane technique (Masquelet’s technique, a Masquelet, the induced membrane technique, induced membrane) is a two-step process that can be used to stimulate high-quality bone regeneration even for massive (>15cm) bone loss or other defects . While it is also commonly used to lengthen short but otherwise healthy limbs, the current discussion focuses on defects resulting from trauma, infection or excision of tumors.
Step 1: inducing the formation of a membrane
Although Masquelet first described this technique in 1986, it wasn’t tested in humans until 2003. This procedure’s major advancement is that it manipulates the patient’s immune system to promote rapid bone regeneration.
As part of the immune reaction to the presence of polymethyl methacrylate (PMMA, bone cement), a bone growth-stimulating membrane will form on the cement’s outer surface. Masquelet’s major innovation was in removing this membrane from the cement it grew on and using it to stimulate and support the amalgamation and vascularization of grafted bone tissue into stable bone.
Assuming no major debridement of the bone becomes necessary to prepare for the graft, the spacer is the same length as the bone segment that must be replaced; therefore, the membrane will be the size and shape needed to surround the necessary graft. In addition to inducing the membrane’s formation and setting its size, this spacer helps stabilize the area affected by the defect. Fixation (either internal, e.g., plating, or external, e.g., a ring fixator) can also be applied as necessary. In most cases, the limb can return to limited weight-bearing until the bone graft (step 2). Over the next six to eight weeks, a vascular membrane will coat the spacer. This should also allow enough time for soft tissue to recover. If not, the surgeon may choose to wait longer.
Step 2: Placing the bone graft
When the surgeon is confident that the surgical site and surrounding soft tissue are ready, the site can be re-opened, the cement spacer removed and detached from the membrane, and the graft material put in place. Although allografting can give good results, grafts are ideally autologous. The most common donor sites are the iliac crest and the intramedullary canal (via reaming).
To contain and shape the graft, Bernstein mentions various methods, e.g., 3D-printed lattices, cages or baskets. However, owing to the shortage of adequately-powered studies, current use is based more on personal preference than on empirical evidence.
Even for grafts longer than 15cm, the Masquelet technique offers a mean recovery period of eight to twelve months. While this is slow for a normal fracture, it is far quicker than distraction osteogenesis (DO) for longer defects. Whereas the Masquelet technique’s healing time is independent of the defect length, DO healing time grows exponentally as that length increases. However, DO also has a significantly higher success rate. Whereas DO’s first-intervention success rate is 95%, that of the Masquelet technique is 86%. With revision, this rises to 90%. Still, even considering that DO’s advantage reflects decades more study and adjustment, this difference cannot be ignored.
II. Avoiding Complications
Although it may be years before Masquelet’s technique can match DO’s success rate, its complication rate is already much lower. This is largely because, overall, Masquelet’s technique is a much simpler procedure.
At the same time, being simpler does not make it applicable to all situations. As Bernstein notes, while many demonstrations showcase Masquelet’s technique on massive femoral defects, it is difficult to reproduce that level of success in the distal tibia, which entails a specific set of challenges.
This agrees well with Lee’s comment that “the distal tibia is a specifically hostile area because it's just not great muscle coverage around there and maybe just terminal vascularity. And so I think…[Morris et al.’s] paper is a wake-up call. I thought it was great.”
In this part of the webinar, Bernstein and Lee agree that, regarding the specific challenges of working with tibial defects, Morris et al. (2017) provide an unvarnished depiction of tibial reconstruction and its risks.
In Morris et al.’s sample, eight of their twelve cases involved defects of the diaphysis, three of the distal tibia, and one of the tibial plateau/diaphysis. Five applications of the induced membrane technique resulted in union. Of these, three involved no complications, i.e., the induced membrane formed as hoped and the patient progressed without further measures or complications. Of the remaining two patients from this subset, one was also fitted with a Taylor space frame (TSF); and in the final case, stage 2 was delayed by 40 days because of infection.
Complications and their possible contributing factors
Of the seven cases where the induced membrane technique was unsuccessful, two led to successful union after mechanical fastening. In a third, which was still underway at the time of Morris et al.’s article, distraction osteogenesis and IM nailing had been applied. Two more were delayed for long periods. In the first of these, a plate breakage after two years revealed incomplete union; and in the second, exchange nailing was necessary. In that case, infection delayed a full union but did not prevent the filling of the defect. And in the most problematic two cases (both of whom were smokers), severe infection led to below-knee amputations.
While Morris et al.’s small sample lacked the strength to produce significant differences, infection coincided with slow, incomplete or failed recovery: all five cases noting infection were delayed because of it . And while one infection could be treated, leading to union, two were delayed for more than one year, and two more, as noted, led to amputation. Smoking and/or recreational drug use may also have contributed to induced membrane failures: Five of the seven cases where that technique was unsuccessful, including both that led to amputation, coincided with smoking.
To avoid complications, then, the most obvious strategy would be to monitor closely for infection. Where it occurs, or where a graft fails, Masquelet et al. (2019) recommend thorough debridement before installation of a spacer. As an extra measure of insurance, Bernstein also includes antibiotics in his spacers’ PMMA.
A nuanced approach is essential
Of course, not all possible complications are directly related to Masquelet’s technique. It is still necessary, for example, to weigh out patient characteristics including age, soft tissue condition, smoking status and any morbidities.
Whatever the surgeon’s treatment plan is, though, it’s necessary to go into the OR with a plan that’s clear but still flexible enough to respond to the situation as it unfolds. In Canada, where hospitals are government-run, OR time is at a premium. For Bernstein, that means waiting until the patient’s soft tissue can handle surgery, then booking an OR. Compared with the danger of ignoring soft tissue needs, a few more weeks won’t make a major difference.
Once part 2 of the strategy is underway, of course, the surgeon must be prepared for every foreseeable need. Even where perfection isn’t possible, progress usually is.
Similarly, particularly in cases of smaller defects, installing a cement spacer at the time of the initial surgery doesn’t necessarily commit the surgeon to a Masquelet technique. Even if no (useable) membrane forms, the spacer will hold the bone ends apart and allow at least limited weight-bearing. When the time comes for step 2, the spacer will be removed, leaving an open space for the bone graft. Until then, the site will be well-stabilized and the soft tissue will have time to heal.
After fully stabilizing the site and waiting for around two months, the next sub-project is to fill the defect. Even here, the surgeon has considerable flexibility. While autologous grafts are normally ideal, not all patients can spare the necessary tissue. In those cases, allografts—whether of sterilized, non-viable bone or even synthetic material—can offer the necessary conditions for osteogenesis.
On the other hand, even in dealing with defects, Lee advocates against committing automatically to inducing a membrane, as a cement spacer could effectively block the patient’s natural healing mechanisms from taking effect. “Sometimes people are so determined to use this technique that they take away any possible chance of the defect decreasing in size or actually harm the tissue.”
And finally, in terms of scheduling the bone graft, Lee advises a case-by-case approach.
I think those peaks [i.e., optimal reported timing for grafts] are probably different in every patient. This idea of using a specific time, to me is a little challenging at this point because we don't really know where the peaks are necessarily. And so to me, it's all about when that soft tissue looks optimal, when I'm convinced there's not a hidden infection in there. I just wait until I really think the soft tissue bed is really well healed (Lee, 27:27).
Ilizarov’s fixation techniques are recommended as a strong choice
Lee’s second case study focuses on multiple failed attempts to correct a defect resulting from an infection-related resection. This leads to two important questions. First, there is the stability of the surgical site. Both Lee and Bernstein agree that the fixation of the defect and associated structures needs to be stable enough to weather a long healing period. Therefore, Bernstein recommends at least two-column fixation. He also mentions that he often uses Ilizarov’s external fixation techniques, which he finds extremely stable.
The second question asks how BMP-2, which stimulates growth in living cells, works with allografts, which have no living cells . Lee answers this question quite concisely:
Allograft doesn't have cells but one thing that people need to realize is that allograft is actually a reasonable adherence environment for cells that migrate into that area. And so when we put BMP plus allograft, it's actually not a completely unreasonable idea because you're actually providing an osteoconductive zone for cells to migrate into, and the signal for cells to migrate into the area (Lee, 40:45).
III. Advanced techniques
Transport nail and distraction osteogenesis
Lee’s third case study highlights a case where, for a short tibial defect, he decided to use a transport nail and distraction osteogenesis instead of Masquelet’s technique. While Bernstein notes that this would be prohibitively expensive for many hospitals, he also praises it as “aggressively intelligent” and highly effective. Lee supports this evaluation with a video of the patient walking well after only three months.
Use of a tantalum implant
The fourth case demonstrates an equally intelligent solution that borrows from oncology by allografting two short pieces of tantalum—taken from a total knee replacement pack—into the defect site. This segment describes both an advanced technique for bone regeneration in the distal tibia and a unique approach to bone defect reconstruction in a challenging case (fulfilling learning goals 3 and 4). Lee’s video of the patient literally dancing on his reconstructed tibia emphasizes what Bernstein speaks of as the psychosocial effects of surgery.
IV. A unique approach
Storage of a large bone fragment for later autotransplantation
While Lee’s third and fourth cases show exemplary skill, his fifth and final example deals particularly impressively with a high-grade open tibial fracture with a large medial-column fragment. At the time of his initial involvement, the patient’s medical status would not permit reconstructive surgery. Still, if left in place, the fragment would likely create serious problems. Borrowing this time from neurosurgical technique, Lee sequestered the fragment in a pouch in the patient’s thigh. Five months later, when the patient’s other injuries had recovered sufficiently, that fragment could be retrieved and autografted as necessary. Two months later the patient was bearing weight and on a path to recovery.
AO Trauma is grateful to Bernstein and Lee for sharing their extensive experience and insights in this webinar.
Watch a video recording of the original AO webinar:
About the authors
Mitchell Bernstein is an Associate Professor of Orthopaedic Surgery at McGill University Health Center in Montreal Canada. He specializes in acute and late fracture reconstruction. He has special expertise in limb lengthening and deformity correction surgery. He is the residency program director at McGill University in Orthopaedic Surgery.
Recommended reading:
- Alford AI, Nicolaou D, Hake M, McBride-Gagyi S. Masquelet's induced membrane technique: Review of current concepts and future directions. J Orthop Res. 2021 Apr;39(4):707-718. doi: 10.1002/jor.24978. Epub 2021 Jan 13. PMID: 33382115; PMCID: PMC8005442.
- Roddy E, Debaun MR, Daoud-gray A, et al. 2017. Treatment of critical-sized bone defects: clinical and tissue engineering perspectives. Eur. J. Orthop. Surg. Traumatol 1. [Google Scholar] [Ref list]. Cited in Alford et al. 2021
- Masquelet A, Kanakaris NK, Obert L, et al. 2019. Bone Repair Using the Masquelet Technique. J. Bone Jt. Surg 101(11):1024–1036. [PubMed] [Google Scholar]. Cited in Alford et al.
- Morris R, Hossain M, Evans A, Pallister I. Induced membrane technique for treating tibial defects gives mixed results.
- Bernstein M, Fragomen AT, Sabharwal S, Barclay J, Rozbruch SR. Does Integrated Fixation Provide Benefit in the Reconstruction of Posttraumatic Tibial Bone Defects?
Clin Orthop Relat Res. 2015 Oct;473(10):3143-53. doi: 10.1007/s11999-015-4326-6.PMID: 25940337 Free PMC article. - Bernstein M, Fragomen A, Rozbruch SR. Tibial Bone Transport Over an Intramedullary Nail Using Cable and Pulleys. JBJS Essent Surg Tech. 2018 Mar 28;8(1):e9. doi: 10.2106/JBJS.ST.17.00035. eCollection 2018 Mar 28.PMID: 30233981 Free PMC article.
You might also be interested in:
AO Surgery Reference
AO Surgery Reference is your go-to resource for the management of fractures, based on current clinical principles, practices, and available evidence.
myAO
Join AO's digital network! On myAO, you can securely save and share cases, connect and exchange knowledge with peers, and access leading clinical and scientific expertise.
AO video hub
Discover a wealth of educational videos, practical exercises, recorded webinars, expert presentations, and interviews from AO's world-renowned faculty.