Osteoporosis: The Silent Killer
BY ROBERT MCGUIRE, MARTIN STODDART, JUERG GASSER, AN SERMON, AND BOYKO GUEORGUIEV
A rapidly growing global problem
Osteoporotic fractures occur every three seconds worldwide, affecting 8.9 million people yearly. Among people over 50, 15% of women and 10% of men are vulnerable to such fractures, creating a massive strain not only on the people it affects directly, but also on national healthcare systems. In the US alone, osteoporotic fractures currently cost 14 billion USD per year. By 2025, that number is expected to more than double.
The reason we call osteoporosis a silent killer is that it causes fractures that often go undetected, but significantly increase mortality risk. Hip fractures correlate with a mortality risk of 6.7%, but are almost always diagnosed and corrected; spinal fractures accompany an 8.6% risk, but usually go undetected. Both of these mortality figures represent roughly 30% increases in one-year mortality. Therefore, it is vital that we are all aware of ways to stabilize or even prevent these injuries.
The Pathophysiology of the Aging Skeleton
As we know, women are much more likely than men to develop osteoporosis. This is linked to post-menopausal hormonal changes, which lead to accelerated bone loss. Following menopause, bone loss is caused predominantly by a negative bone balance in each bone remodeling cycle, combined with an increase in remodeling events. This is not simply a matter of the bones thinning. Resorption on trabecular surfaces also creates local stress concentrators. Therefore, a bone loss of 10% will increase fracture risk several-fold. So, while the risk of fractures increases with age in both genders, it is roughly 50% more prevalent in post-menopausal women.
The most common osteoporotic fractures are of the vertebrae and hip, both of which carry weight, and of the wrist, which tends to bear the shock of falls. For hip fractures, the hospitalization rate is 100%; however, fewer than 11% of vertebral fractures lead to hospital stays. Still, relative survival rates for hip and vertebral fractures are very similar—respectively .83 and .82. Both represent an increase of roughly 30% over people without fractures.
Of course, mortality rates tell only part of this story. Both hip and vertebral fractures also greatly reduce patients’ quality of life. Still, between 60 and 85% of women who suffer these fractures receive no osteoporotic drug treatment.
The most effective medical treatment options target two cellular processes involved in bone mass regulation: bone remodeling and bone modeling.
Bone remodeling is a continuous maintenance function. Osteocytes detect and repair microdamage and generally renew tissues. However, as people age, their systems tend to remove cells more quickly than they replace them, leading to net bone loss. Bone resorption is the single greatest direct contributor to osteoporotic bone weakening.
Modeling, on the other hand, is an adaptive but normally short-lived process. It quickly increases bone strength and maintains mechanical competence via osteocyte production. Each osteocyte has a strength sensor, which is mainly active during skeletal growth. However, it can also be triggered by anabolic therapies such as parathyroid hormone (PTH), which leads to bone growth. This is commonly combined with sclerostin inhibitors, which slow bone resorption.
A four-year RCT of all approved antiresorptive/ PTH therapies showed very strong relative risk reduction regarding new vertebral fractures with the use of zoledronic acid (Zoledronate, a bisphosphonate) (-70%), Denosumab (-68%), Teriparatide 20µg (-65%) and Ibandronate 2.5 (-62%). A primary trial in hip fracture relative risk was inconclusive; however, meta-analyses show that PTH therapies have a potent preventive effect against hip fracture as well.
How bisphosphonate therapies work
Bisphosphonates can be administered either orally or intravenously. They are preferentially incorporated into mineralized bone surfaces. By blocking the prenylation of small GTPase signaling proteins, they slow bone resorption until they are removed from the bone surfaces via remodeling. This means they can provide several years of protection.
Just as mortality rates rise with fractures, they fall with protective therapies. In a 2007 RCT of zoledronic acid, Lyles et al. found that fracture risk in the treatment group was 30% lower than in the control group, with an accompanying improvement of 28% in all-cause mortality. So an effective protective therapy both decreases mortality and increases quality of life.
Long-term treatment is limited
One important limitation of these therapies is that, although they are very effective, their unwanted side effects will eventually outweigh their benefits. Therefore, depending on the drug class, maximum recommended uninterrupted treatment times range from twelve months to six years (although some patients manage well for up to ten years). After stopping bisphosphonates, the patient should take a drug holiday of up to three years before reinitiating them. For other drug groups, bisphosphonates are commonly used to bridge gaps in protection. For example, stopping anabolic treatment can lead to rapid bone loss. Switching to bisphosphonates prevents that, thereby protecting the first treatment’s benefits.
Management of Vertebral Body Structures
Vertebral fractures
As we noted above, hip fractures are immediately recognizable as medical emergencies that require hospitalization. For vertebral fractures, however, most are never even diagnosed: only 22–33% ever become clinically evident. However, for people who experience them, mortality rates increase hugely—more than four times that of people without fractures, and double that of age-matched controls. The five-year survival rate for untreated vertebral fractures is 30%.
A sentinel event
In fact, in cases where a vertebral fracture is painful enough that a person seeks medical help, that fracture is often the patient’s first sign that they have osteoporosis. Therefore, where such fractures occur in otherwise healthy people, clinicians should be diligent in evaluating overall bone health.
The role of vitamin D deficiency
Vitamin D is vital to good bone health, but deficiency is extremely common. The majority of women with osteoporosis, and 90% of women over 70 are vitamin D-deficient. From the perspective of a physician treating osteoporotic fractures, low vitamin D levels can slow or even prevent skeletal healing. So before performing surgery, it is a very good idea to check the patient’s vitamin D levels and correct it if necessary.
Treatment leads to significant benefits
Compared to unoperated patients, those who receive surgery for vertebral fractures have a 20% greater five-year survival rate (i.e., 60.8% for operated patients vs. 50% for unoperated). Those operated within one or two days of admission have the highest recovery rates. Therefore, quick and rather aggressive treatment is recommended.
The most common treatment is vertebroplasty. This involves pumping in low-viscosity bone cement to fill in the fracture’s interstices. In other cases, a kyphoplasty is more appropriate. For that, the surgeon uses a stent or balloon to create a cavity, then fills that with cement.
Vertebroplasty versus kyphoplasty
While both vertebroplasty and kyphoplasty are highly effective, both entail risks. As vertebroplasty involves pumping cement into the bone under pressure, it is possible to extrude the cement into the canal or lead to embolic phenomena. And with a kyphoplasty, it is possible to rupture part of the vertebral body. So both require careful application.
Still, even while bones heal well with both surgeries, they heal with osteoporotic bone. And because that is very soft, screwing into it is much like screwing into butter. To provide holding power, it is usually necessary to anchor the screws in cement injected behind the bone.
Use of fenestrated screws
Fenestrated screws are hollow, allowing a path through which to pump in cement. The object is not to fill the cavity behind the screw completely, but to form a ball of cement on the end of the screw. This greatly increases the area supporting the screw. If a kyphoplasty is indicated, the surgeon can insert a stent or balloon, fill it with cement, and screw into the cement.
Anterior stabilization is also commonly necessary.
For both vertebroplasty and kyphoplasty, which are posterior instrumentation solutions, it is often necessary to add anterior stabilization. For that, the surgeon removes the fractured vertebral body and places a stabilizer anteriorly to reconstruct the anterior column. Because of the characteristics of osteoporotic bone, this can only be used in addition to—not instead of—posterior instrumentation.
Long anatomic constructs
Long anatomic constructs are sagitally balanced implants that help avoid problems with pullout and proximal junctional kyphosis. Ideally, these implants will allow restoration of spinal balance with minimally invasive surgery. That is, with one small incision, the surgeon can add multiple levels of fixation.
Use of anabolic therapies
In all of these cases, anabolic drugs can be used to strengthen the bone and protect against future injuries or deterioration. Ideally, these can be administered well before an elective surgery such as a stenosis to increase the bone stability. This increases the strength not only of the construct, but of the bone itself, protecting against further fractures.
Proximal junctional kyphosis (PJK)
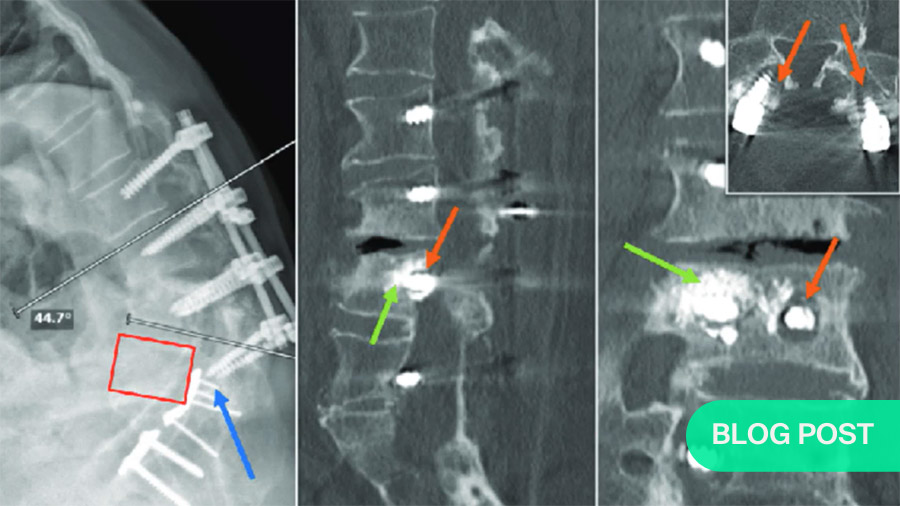
With all the benefits of strengthening the damaged area of the spine, it is also very important to remember that the vertebrae above the implant-strengthened section remain very vulnerable. As this often results in progressive kyphosis, particularly in the thoracic vertebrae, there is a significant risk of developing neurologic compromise. To guard against this, we can inject various amounts of cement into the at-risk vertebrae to distribute the stresses. In terms of fracture prevention, tapering the doses, i.e., 4ml, then 3ml then 2ml between successive vertebrae, has shown an excellent protective effect against fractures.
Problems with treatment
Therapies to enhance the quality of the bone with anabolic medications and neuropeptide Y (NpY) antagonists are very promising. Augmentation of our fixation gives us opportunities first to be able to stabilize these fractures in acute phases, and second to assist in stabilizing our elective patients. When stabilizing vertebral fractures, though, it is vital to create anatomical alignment as well. Otherwise, the risk of implant failure is much higher.
Osteoporotic long bone fractures
As with vertebral fractures, osteoporotic long bone fractures occur because of a combination of decreased bone mass and weakened and brittle bone architecture. When repairing these types of fractures, we also often have to deal with implants present from earlier surgeries.
Regarding the epidemiology of osteoporotic long bone fractures, two findings are particularly interesting. First, it is not usually the shafts of the involved bones that fracture, but the articulations. Younger geriatric patients tend to have osteoporotic wrist and proximal humerus fractures; older ones tend to break their hips. Second, a prior fracture is a significant risk factor for further fractures, especially in the immediate post-fracture period. This is very important when considering treatment options.
Need for adjusted implants
The low bone mass of osteoporotic bones leads to a difficult implant anchorage. In hips, this can lead to cut-out of the implant and secondary varisation of the femoral head. In other locations like the proximal humerus, loosening of implants can occur. To avoid this problem, we need solutions to increase implant anchorage. New implant designs requiring no drilling like helical blades and barrels, provide better anchorage of the implant. Angular-stable plates and screws, as well as angular stable locking screws (ASLS) for use with nails are also very useful options. Finally, as with vertebral fractures, implant augmentation with bone cement increases the implant to bone surface leading to a better anchorage .
In case of shaft fractures, previous implants like prostheses or osteoynthesis materials are often present. In fact, in recent years there has been an explosion in the incidence of periprosthetic fractures. These can be quite challenging to repair, but currently-available modular revision prostheses and locking attachment plates can all be very useful to stabilize periprosthetic and peri-implant fractures.
Avoiding periprosthetic and secondary fractures
Because people who receive implants spanning only parts of long bones are prone to later fractures just next to the implant, it might be better to prophylactically treat an initial fracture with a full-length implant. In light of the shortfalls of existing implants, for example, in buttressing an unfractured femoral neck, complete femur spanning implants are really what is necessary, but are not yet available on the market.
In long bones, it is common for an initial “sentinel” fracture to be followed by a secondary fracture within one year. For this reason it is good to set a very low threshold for surgical treatment and to treat osteoporotic fractures as early and aggressively as you can by spanning complete bones and allowing for immediate full weight bearing. This will help patients in regaining their mobility and avoiding falls causing secondary fractures.
Post-surgical care and management
One-shot surgery should be the goal, followed by immediate mobilization and weight-bearing as tolerated. It is also important to consider the patient’s overall health, especially co-morbidities, which are often present in geriatric patients. The care team has to collaborate with the patient’s geriatrician to co-manage care, along with creating fracture liaison services. These work cost-effectively to detect and treat osteoporosis. They also offer fall prevention programs. As osteoporotic fracture patients are often frail, plaster casts or bulky bandages should be avoided, as these can increase the risk of falls.
Summary
For long bone osteoporotic fractures, the main recommendations are not to underestimate the first fracture and to initiate a timely, aggressive and functional surgical treatment for obtaining optimal results. Ideally, implant constructs should be chosen that allow for immediate mobilization and full weight-bearing.
An ounce of prevention is worth a pound of cure: Prophylactic strategies regarding osteoporotic fractures
One major question about osteoporotic fractures is that of which strategies are most effective at preventing them.
Tools to measure osteoporotic fracture risk
Before we can answer that question, we need some idea of who is at risk of fracture and how to assess their risks. Simpler possibilities include DXA measurements or FRAX scores for 10-year probability of osteoporotic fractures. To gauge the two-year risk of spine, hip or wrist fractures, finite element analysis (FEA) involves more complex data. In addition to bone characteristics including density and mineral content, FEA also gauges the strains and stresses that occur under diverse loading scenarios. The most important of these scenarios is accidental loading.
Fractures occur when the load on a bone or joint is greater than the bone competence. Except in extreme cases of osteoporosis, that means an accident, especially a fall. In fact, the fracture risk is the ratio of accidental load over bone competence. Therefore, the paths to protection are to prevent accidental overloading, either by preventing falls or by minimizing their effects, for example, by cushioning them. However, as a matter of patient safety, such risks are impossible for clinicians to manage.
They can, however, increase the bone competence. Strategies include the medications described above, which activate the body’s own systems to strengthen the bone, or mechanical augmentation such as cement or metal implants.
All of these protective measures are already in common use where fractures occur. As a result, bones with reduced fractures are typically well-protected. The next step is to use augmentation not only correctively, but also prophylactically. That is, if we identify significant risks in uninjured bones, it makes sense to augment them without waiting for a fracture.
Naturally, before proceeding further, any prospective prophylactic solutions need to be developed via virtual and ex vivo testing. Virtual biomechanical bone modeling results currently not only correlate closely with those of mechanical experimentation, they also provide additional data. For example, computer modeling can predict how stresses will be redistributed after an augmentation. This allows us to predict the most likely locations of secondary fractures.
Barriers to prophylactic treatment
Unfortunately for highly osteoporotic patients, we can currently only reduce the risk of further fractures around injuries. Ethical concerns prevent us from operating on weak but uninjured areas. Further considerations include whether proposed prophylactic measures are clinically feasible, biologically safe and mechanically sufficient. For the moment, then, although prophylactic augmentation shows eminent clinical potential, no biomechanical solutions are yet available. Even if they were, there are no clear indications for their use, and ethical justifications remain unclear. Still, research is continuing.
As noted above, regarding the use of bone cement to augment implants, it is clear that it smooths the transition between the bone and the implant, providing a larger bone-implant contact area, and better implant anchorage, leading to better stability of the fixed structure.
The joints with the greatest biomechanical potential for osteoporotic stabilization are the shoulder, the vertebrae, the knee and the hip. However, we are not advocating routine use of prophylaxis for these sites. The benefits must be weighed against the risks on a case-by-case basis. Applied responsibly, though, the concept appears safe.
An ounce of prevention
Finally, assuming that the current ethical and technical barrier can be overcome, implementation of prophylactic treatment of osteoporotic patients would remain problematic in areas where healthcare compensation is based on reactive treatment of acute conditions. However, like vaccinations, preventive care of osteoporotic conditions is an investment that will more than pay for itself in terms both of saved long-term costs and of patients’ ongoing quality of life. Until then, the pharmaceutical and surgical therapies and augmentation strategies described here for osteoporotic fractures are an excellent start.
AO sincerely thanks the authors for contributing their time and expertise to this webinar series.
Summary:
This blog accompanies and summarizes the AO webinar titled “Osteoporosis—The Silent Killer” presented by Robert McGuire and Martin Stoddart. Originally broadcasted live, it featured interactive telepresentations by four top scientists and surgeons in this field: Jürg Gasser, Robert McGuire, An Sermon, and Boyko Gueorguiev. Topics include effective medications, fixation techniques for affected and at-risk vertebrae and long bones, as well as prospects for prophylactic treatment.
Watch a video recording of the original AO webinar:
About the authors
All of the participants in this discussion are experts in their respective fields of research and surgery with many years of experience as it relates to osteoporosis.
Robert McGuire is the immediate past president of the AO foundation. He is an orthopedic spine surgeon at the University of Mississippi Jackson, Mississippi.
Martin Stoddart is a principal scientist and the regenerative orthopedic program leader at the AO Research Institute in Davos.
Jürg Gasser is an associate director in the musculoskeletal disease (MSD) area at Novartis Institutes for Biomedical Research in Switzerland. He was the co-inventor and developer of zoledronic acid and has worked extensively with pharmacological treatment options for osteoporosis.
Boyko Gueorguiev is the leader of biomedical development at the AO research Institute in DeVos, Vice President of the European Orthopedic Research Society, and Honorary Member of both the Bulgarian Orthopedic & Traumatology Association and the Serbian Trauma Association. He is Academic Council Member of the University Multiprofile Hospital for Active Treatment and Emergency Medicine 'N I Pirogov', Sofia, and Honorable Research Fellow of the Institute of Metal Science ('Acad A Balevski') at the Bulgarian Academy of Sciences.
References
- T. J. de Villiers & S. R. Goldstein (2022) Bone health 2022: an update, Climacteric, 25:1, 1-3, DOI: 10.1080/13697137.2021.1965408
Further suggested readings
-
By Robert McGuire:
- Holroyd C, Cooper C, Dennison E. Epidemiology of osteoporosis. Best Pract Res Clin Endocrinol Metab. 2008;22(5):671-685.
- Johnell O, Kanis JA, Oden A, et al. Mortality after osteoporotic fractures. Osteoporos Int. 2004;15(1):38-42.
- Lee HM, Park SY, Lee SH, Suh SW, Hong JY. Comparative analysis of clinical outcomes in patients with osteoporotic vertebral compression fractures (OVCFs): conservative treatment versus balloon kyphoplasty. Spine J. 2012;12(11):998-1005.
- Zhang J, He X, Fan Y, Du J, Hao D. Risk factors for conservative treatment failure in acute osteoporotic vertebral compression fractures (OVCFs). Arch Osteoporos. 2019;14(1):24.
- Hoshino M, Tsujio T, Terai H, et al. Impact of initial conservative treatment interventions on the outcomes of patients with osteoporotic vertebral fractures. Spine (Phila Pa 1976). 2013;38(11):E641-648.
- Park HY, Ahn JH, Ha KY, et al. Clinical and radiologic features of osteoporotic spine fracture with delayed neurologic compromises. World Neurosurg. 2018;120:e1295-e300.
- Tsujio T, Nakamura H, Terai H, et al. Characteristic radiographic or magnetic resonance images of fresh osteoporotic vertebral fractures predicting potential risk for nonunion: a prospective multicenter study. Spine (Phila Pa 1976). 2011;36(15):1229-1235.
- Wakao N, Takeuchi M, Riew DK, et al. Effect of an intensive conservative therapy with daily teriparatide administration and rehabilitation for osteoporotic delayed vertebral collapse and paralysis. Medicine (Baltimore). 2018;97(23):e10906.
- Kao FC, Huang YJ, Chiu PY, Hsieh MK, Tsai TT. Factors predicting the surgical risk of osteoporotic vertebral compression fractures. J Clin Med. 2019;8(4):501.
- Ha KY, Park KS, Kim SI, Kim YH. Does bisphosphonate-based anti-osteoporosis medication affect osteoporotic spinal fracture healing? Osteoporos Int. 2016;27(2):483-488.. Muehlematter UJ, Mannil M, Becker AS, et al. Vertebral body insufficiency fractures: detection of vertebrae at risk on standard CT images using texture analysis and machine learning. Eur Radiol. 2019;29(5):2207-2217
-
By Jürg Gasser:
- Sambrook P, Cooper C. Osteoporosis. Lancet. 2006 Jun 17;367(9527):2010-8. doi: 10.1016/S0140-6736(06)68891-0. Erratum in: Lancet. 2006 Jul 1;368(9529):28. PMID: 16782492.
D Bliuc et al, Mortality Risk Associated With Low-Trauma Osteoporotic Fracture and Subsequent Fracture in Men and Women. JAMA. 2009;301(5):513–521. doi:10.1001/jama.2009.50 - Cauley JA, Thompson DE, Ensrud KC, Scott JC, Black D. Risk of mortality following clinical fractures. Osteoporos Int. 2000;11(7):556-61. doi: 10.1007/s001980070075. PMID: 11069188.
- Van Der Linden, J.C., Homminga, J., Verhaar, J.A.N. and Weinans, H. (2001), Mechanical Consequences of Bone Loss in Cancellous Bone. J Bone Miner Res, 16: 457-465. https://doi.org/10.1359/jbmr.2001.16.3.457
- Power, J., Loveridge, N., Lyon, A., Rushton, N., Parker, M. and Reeve, J. (2003), Bone Remodeling at the Endocortical Surface of the Human Femoral Neck: A Mechanism for Regional Cortical Thinning in Cases of Hip Fracture. J Bone Miner Res, 18: 1775-1780. https://doi.org/10.1359/jbmr.2003.18.10.1775
- Gasser, J.A., Kneissel, M. (2017). Bone Physiology and Biology. In: Smith, S., Varela, A., Samadfam, R. (eds) Bone Toxicology. Molecular and Integrative Toxicology. Springer, Cham. https://doi.org/10.1007/978-3-319-56192-9_2
- Bousson, V., Meunier, A., Bergot, C., Vicaut, É., Rocha, M.A., Morais, M.H., Laval-Jeantet, A.-M. and Laredo, J.-D. (2001), Distribution of Intracortical Porosity in Human Midfemoral Cortex by Age and Gender. J Bone Miner Res, 16: 1308-1317. https://doi.org/10.1359/jbmr.2001.16.7.1308
- Delmas PD. Treatment of postmenopausal osteoporosis. Lancet. 2002 Jun 8;359(9322):2018-26. doi: 10.1016/S0140-6736(02)08827-X. PMID: 12076571.
- Rogers MJ, Frith JC, Luckman SP, Coxon FP, Benford HL, Mönkkönen J, Auriola S, Chilton KM, Russell RG. Molecular mechanisms of action of bisphosphonates. Bone. 1999 May;24(5 Suppl):73S-79S. doi: 10.1016/s8756-3282(99)00070-8. PMID: 10321934.
- Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J,
- Hue TF, Sellmeyer D, Eriksen EF, Cummings SR; HORIZON Pivotal Fracture Trial. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007 May 3;356(18):1809-22. doi: 10.1056/NEJMoa067312. PMID: 17476007.
- Lyles KW, Colón-Emeric CS, Magaziner JS, Adachi JD, Pieper CF, Mautalen C, Hyldstrup L, Recknor C, Nordsletten L, Moore KA, Lavecchia C, Zhang J, Mesenbrink P, Hodgson PK, Abrams
- K, Orloff JJ, Horowitz Z, Eriksen EF, Boonen S; HORIZON Recurrent Fracture Trial. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007 Nov 1;357(18):1799-809. doi: 10.1056/NEJMoa074941. Epub 2007 Sep 17. PMID: 17878149; PMCID: PMC2324066.
- Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009 Aug 20;361(8):756-65. doi: 10.1056/NEJMoa0809493. Epub 2009 Aug 11. Erratum in: N Engl J Med. 2009 Nov 5;361(19):1914. PMID: 19671655.
- Kraenzlin ME, Meier C. Parathyroid hormone analogues in the treatment of osteoporosis. Nat Rev Endocrinol. 2011 Jul 12;7(11):647-56. doi: 10.1038/nrendo.2011.108. PMID: 21750510.
- Greenspan SL, Bone HG, Ettinger MP, Hanley DA, Lindsay R, Zanchetta JR, Blosch CM, Mathisen AL, Morris SA, Marriott TB; Treatment of Osteoporosis with Parathyroid Hormone Study Group. Effect of recombinant human parathyroid hormone (1-84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med. 2007 Mar 6;146(5):326-39. doi: 10.7326/0003-4819-146-5-200703060-00005. PMID: 17339618.
- Lindsay R, Cosman F, Zhou H, Bostrom MP, Shen VW, Cruz JD, Nieves JW, Dempster DW. A novel tetracycline labeling schedule for longitudinal evaluation of the short-term effects of anabolic therapy with a single iliac crest bone biopsy: early actions of teriparatide. J Bone Miner Res. 2006 Mar;21(3):366-73. doi: 10.1359/JBMR.051109. Epub 2005 Nov 21. PMID: 16491283.
- Dempster DW, Cosman F, Kurland ES, Zhou H, Nieves J, Woelfert L, Shane E, Plavetić K, Müller R, Bilezikian J, Lindsay R. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res. 2001 Oct;16(10):1846-53. doi: 10.1359/jbmr.2001.16.10.1846. PMID: 11585349.
- Black DM, Bilezikian JP, Ensrud KE, Greenspan SL, Palermo L, Hue T, Lang TF, McGowan JA, Rosen CJ; PaTH Study Investigators. One year of alendronate after one year of parathyroid hormone (1-84) for osteoporosis. N Engl J Med. 2005 Aug 11;353(6):555-65. doi: 10.1056/NEJMoa050336. PMID: 16093464.
- Eastell R, Nickelsen T, Marin F, Barker C, Hadji P, Farrerons J, Audran M, Boonen S, Brixen K, Gomes JM, Obermayer-Pietsch B, Avramidis A, Sigurdsson G, Glüer CC. Sequential treatment of severe postmenopausal osteoporosis after teriparatide: final results of the randomized, controlled European Study of Forsteo (EUROFORS). J Bone Miner Res. 2009 Apr;24(4):726-36. doi: 10.1359/jbmr.081215. PMID: 19049337.
- Lewiecki EM, Blicharski T, Goemaere S, Lippuner K, Meisner PD, Miller PD, Miyauchi A, Maddox J, Chen L, Horlait S. A Phase III Randomized Placebo-Controlled Trial to Evaluate Efficacy and Safety of Romosozumab in Men With Osteoporosis. J Clin Endocrinol Metab. 2018 Sep 1;103(9):3183-3193. doi: 10.1210/jc.2017-02163. PMID: 29931216.
- Miller PD, Bolognese MA, Lewiecki EM, McClung MR, Ding B, Austin M, Liu Y, San Martin J. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone. 2008 Aug;43(2):222-229. doi: 10.1016/j.bone.2008.04.007. Epub 2008 Apr 26. PMID: 18539106.
- Tsourdi E, Langdahl B, Cohen-Solal M, Aubry-Rozier B, Eriksen EF, Guañabens N, Obermayer-Pietsch B, Ralston SH, Eastell R, Zillikens MC. Discontinuation of Denosumab therapy for osteoporosis: A systematic review and position statement by ECTS. Bone. 2017 Dec;105:11-17. doi: 10.1016/j.bone.2017.08.003. Epub 2017 Aug 5. PMID: 28789921.
- Cummings, S.R., Ferrari, S., Eastell, R., Gilchrist, N., Jensen, J.-E.B., McClung, M., Roux, C., Törring, O., Valter, I., Wang, A.T. and Brown, J.P. (2018), Vertebral Fractures After Discontinuation of Denosumab: A Post Hoc Analysis of the Randomized Placebo-Controlled FREEDOM Trial and Its Extension. J Bone Miner Res, 33: 190-198. https://doi.org/10.1002/jbmr.3337
- Black DM, Bilezikian JP, Ensrud KE, Greenspan SL, Palermo L, Hue T, Lang TF, McGowan JA, Rosen CJ; PaTH Study Investigators. One year of alendronate after one year of parathyroid hormone (1-84) for osteoporosis. N Engl J Med. 2005 Aug 11;353(6):555-65. doi: 10.1056/NEJMoa050336. PMID: 16093464.
- Sambrook P, Cooper C. Osteoporosis. Lancet. 2006 Jun 17;367(9527):2010-8. doi: 10.1016/S0140-6736(06)68891-0. Erratum in: Lancet. 2006 Jul 1;368(9529):28. PMID: 16782492.
-
By An Sermon:
- Kanis J., Johansson H., Oden A., Harvey N., Gudnason V., Sanders K., Sigurdsson G. et al (2018). Characteristics of recurrent fractures. Osteoporosis international (2018) 29:1747-1757.
- Kanis J., Johansson H., Harvey N., Gudnason V., Sigurdsson G., Siggeirsdottir K., Lorentzon M. et al. The effect on subsequent fracture risk of age, sex, and prior fracture site by recency of prior fracture. Osteoporosis International (2021) 32:1547-1555.
- Bottle A, Griffiths R, White S, et al. Periprosthetic fractures: the next fragility fracture epidemic? A national observational study. BMJ Open 2020;10:e042371. doi:10.1136/ bmjopen-2020-042371
- Borgström F., Karlsson L., Ortstäter G., Norton N., Halbout P., Cooper C. et al. Fragility fractures in Europe, burden, management and opportunities. Arch Osteoporos (2020) 15:59.
- Liem et al. Identifying a standard set of outcome parameters for the evaluation of orthogeriatric co-management for hip fractures. Injury, int. J. Care Injured (2013) 1403-1412
- Deschodt M., Mendelson D., Van Grootven B. Impact of geriatric co-management programmes on outcomse of older surgical patients: update of recent evidence. Current Opinion in Anaesthesiology 2020 33(1):114-121.
- Seys D., Sermon A., Sermeus W., Panella M., Bruyneel L., Boto P., Vanhaecht K. (2018). Recommended care received by geriatric hip fracture patients: where are we now and where are we heading?. Archives Of Orthopaedic And Trauma Surgery, 138 (8), 1077-1087. doi: 10.1007/s00402-018-2939-4.)
- Wilson H. Orthogeriatrics in hip fracture. The Open Orthopaedics Journal (2017) 11 (Suppl-7, M2): 1181-1189.
You might also be interested in:
AO Surgery Reference
AO Surgery Reference is your go-to resource for the management of fractures, based on current clinical principles, practices, and available evidence.
myAO
Join AO's digital network! On myAO, you can securely save and share cases, connect and exchange knowledge with peers, and access leading clinical and scientific expertise.
AO video hub
Discover a wealth of educational videos, practical exercises, recorded webinars, expert presentations, and interviews from AO's world-renowned faculty.