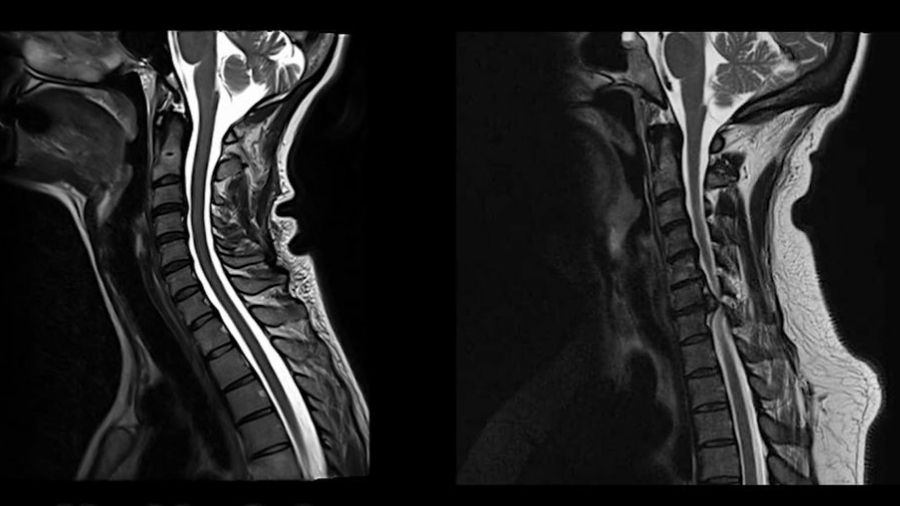
Improving time-to-diagnosis and treatment in degenerative cervical myelopathy: why interdisciplinary teamwork matters
For patients with spine diseases, timely referral to a specialized spine surgeon is essential: often there will be no improvement without surgical intervention, and patients may suffer neurological deterioration in the meantime. But before patients can be assessed by a surgeon, they generally encounter several other healthcare providers. For timely referral, interdisciplinary teamwork between these other healthcare providers and surgeons is critical. To illustrate the value of interdisciplinary teamwork, we take the example of degenerative cervical myelopathy (DCM), a progressive spinal cord injury (SCI) resulting from dynamic and static cord compression and chronic ischemia.
Interdisciplinary teamwork is a key factor needed to foster knowledge about DCM, and, ultimately, to improve patient care. We advocate a stronger collaboration between surgical and non-surgical spine specialists, as well as between spine specialists and other healthcare providers.
Due to lack of awareness and challenges in the differential diagnosis, DCM is often misdiagnosed and therefore not treated in a timely fashion. In fact, the average time to diagnosis of DCM is 2.2±2.3 years, and patients attend an average of 5.2±3.6 physician visits before obtaining a correct diagnosis [1]. This delay in diagnosis results in increased time to surgical management and undoubtedly affects functional and quality-of-life outcomes in these individuals.
Falls as an under-recognized presentation of DCM. In our experience, sadly, patients with undetermined spinal canal stenosis and/or mild DCM signs were referred to surgery only after they fell and sustained more severe neurological injury. In one case, a patient presented to the emergency department following a fall and was evaluated by neurosurgery as her trauma workup was notable for a lumbar compression fracture. She was admitted to medicine for physical therapy evaluation. Upon further questioning, the patient reported progressive gait impairment, recurrent falls, and bilateral hand clumsiness. Physical examination was notable for diffuse hyperreflexia, positive Hoffmann sign, lower extremity spasticity and inability to ambulate. MRI of the cervical spine demonstrated severe cord compression with signal change, consistent with severe DCM.
In a second case, a patient who had previously undergone DCM surgery (10 years ago) presented with significantly worsening functional impairment and recurrent falls. This patient had deteriorated over the last six months to the point where he was mostly bed-bound, incontinent of urine and unable to use his hands to open containers or manipulate a fork or knife. Emergency medical technicians were called three to four times per week to lift him back into his bed after falling. Similar to the first case, his medical history and physical examination were consistent with myelopathy, and an MRI of his cervical spine showed severe cord compression with diffuse signal change. Both of these cases were taken to the operating room for emergency surgical decompression of the cervical spine.
Of note, these individuals had previously presented to the emergency department and their primary care physicians with the chief complaint of recurrent falls, which were attributed to ageing and cognitive disturbances. These cases highlight that recurrent falls may be an indirect indicator of cervical spine compression and that health care practitioners must carry a high degree of suspicion when managing these patients. Multidisciplinary input is required to make an accurate and timely diagnosis of DCM and optimize treatment pathways for these patients.
As part of AO Spine RECODE-DCM, a priority-setting partnership was established in order to reduce research insufficiencies and determine priorities for future investigations into DCM. This initiative identified the Top 10 Research Priorities, taking into consideration the perspectives and interests of key stakeholders, including healthcare professionals, individuals with lived experience and caregivers. The results of the AO Spine RECODE-DCM project highlight the need for multidisciplinary input for the diagnosis and management of DCM.
The following three questions were among those identified as research priorities:
- What strategies can increase awareness and understanding of DCM amongst healthcare professionals and the public? Can these strategies help improve timely diagnosis and management of DCM?
- What are the diagnostic criteria of DCM? What is the role of imaging and when should imaging be used in the assessment of DCM?
- What is the role of rehabilitation following surgery for DCM? Can structured postoperative rehabilitation improve outcome following surgery for DCM? What are the most effective strategies?
Addressing these knowledge gaps requires bridges to be built between spine surgeons and other healthcare professionals, including primary care physicians, rheumatologists, neurologists, physiatrists, and physical therapists.
Establishing diagnostic criteria for DCM
Although spine surgeons are in charge of the surgical treatment of DCM, a diagnosis must be made before a patient can be appropriately referred for specialized surgical evaluation. Within the AO Spine RECODE-DCM community, a diagnostic criteria incubator project is currently underway and has engaged a variety of healthcare providers, individuals with lived experience and caregivers. This multidisciplinary input is essential as
- patients with DCM can provide insight into their first symptoms and the course of their illness prior to diagnosis;
- primary care physicians and non-surgical specialists can outline the more subtle signs and symptoms of DCM that prompted them to obtain further neuroimaging; and
- surgeons can provide their perspectives on ideal surgical candidates and disease components that result in delayed- or misdiagnosis. Multidisciplinary input will ensure that diagnostic criteria are generalizable, that they reflect the different features of the disease and that they accurately identify as many individuals with DCM as possible.
Pioneers in spinal cord neurology and spine surgery, such as Sir Ludwig Guttmann and Charles Elsberg, relied on a detailed neurological examination and patient history while imaging was in its infancy. As a result, patients were more severely impaired when referred to spine surgery. With the dawn of neuroimaging as well as improved access to healthcare, patients are generally referred earlier for MRI in developed countries. MRI is now considered the gold-standard for identifying spinal canal stenosis and detecting structural spinal cord compression. However, given the high prevalence of asymptomatic spinal cord compression [2], poor correlation between imaging findings and disease severity, and a lack of awareness of subtle neurological deficits [3], there is still substantial delay in the diagnosis of DCM and referral to spine specialists.
At early stages, patients with DCM can present with subtle, non-specific symptoms in their upper and lower extremities, making diagnosis challenging. Common complaints include hand numbness, upper extremity weakness, bilateral arm paresthesia, neck pain and stiffness, Lhermitte’s phenomenon, impaired gait, and urgency of urination or defecation. In some cases, the presenting complaint may be non-specific and require further exploration through history.
On clinical examination, patients with DCM exhibit a combination of upper and lower motor neuron signs as well as sensory dysfunction. Upper motor neuron signs include hyperreflexia below the level of the lesion, a positive Hoffmann sign, upgoing plantar responses, lower limb spasticity, corticospinal distribution motor deficits and weakness. Lower motor neuron signs result from compression of the nerve roots as they exit the spinal canal and commonly include atrophy of intrinsic hand muscles, fasciculations and weakness. Patients may also exhibit reduced proprioception or sensation to light touch, temperature, pain, or vibration. Certain clinical signs are not overly sensitive for detecting myelopathy and are not routinely used in clinical practice, especially at the primary care level. As part of the initiative to develop diagnostic criteria for DCM, two systematic reviews will be conducted in order to better define the sensitivity and specificity of various signs and symptoms of myelopathy.
Advanced neurophysiology and neuroimaging of the spinal cord
Cervical spine imaging, specifically MRI, is essential to confirm a diagnosis of DCM. Although spinal canal narrowing is a prerequisite for DCM, the degree of cervical canal stenosis and cord compression is often not associated with disease severity. Carl Zipser is interested in advanced neurophysiology and neuroimaging of the spinal cord. He contributed to a study that investigated spinal cord motion with MRI and determined that movement and ongoing oscillations are increased at the level of maximum stenosis in patients with DCM [4]. This imaging metric holds promise as a biomarker of mechanical stress to the spinal cord and needs to be further studied.
Furthermore, advanced neurophysiological examinations, such as contact-heat evoked potentials (CHEPs), may facilitate the early diagnosis of DCM, and help quantify functional deterioration [5]. Furthermore, Carl Zipser is committed to evaluating cerebrospinal fluid pressure dynamics (CSFP) in patients with acute and chronic spinal cord compression during decompressive surgery (intraoperative monitoring). Bedside intraoperative CSFP monitoring [6–8] may ensure that surgeons completely decompress the spinal cord in a timely manner, inform peri-operative hemodynamic strategies, and quantify post-operative residual cord compression or swelling. Sufficient spinal cord decompression and the preservation of physiological CSFP dynamics are required for effective cell and stem cell applications, which are among the most promising strategies for the treatment of SCI [9]. Bedside CSFP assessments may also have the potential to detect dynamic cord compression in patients with mild DCM. More research is needed to investigate the clinical utility of intraoperative and bedside CSFP assessments.
In summary, diagnosis of DCM is challenging and requires combining information from a detailed medical history, a comprehensive neurological examination and appropriate tests including imaging of the spinal column. Diagnosis also involves multidisciplinary input from physical therapy, non-surgical specialties (e.g., neurology, rheumatology and physiatry) and surgical specialties.
Optimizing non-operative management of DCM
Not all DCM requires surgical management. However, the role of structured nonoperative treatment in stabilizing or improving symptoms in DCM is not well defined. It is unclear what types of conservative measure are the most effective at halting disease progression. Published studies have reported outcomes following a trial of bed rest, cervical traction, cervical immobilization or bracing, thermal therapy, physical therapy, and non-steroidal anti-inflammatory drugs. In the literature, response to nonoperative treatment is typically minimal and not clinically significant. Of note, patients with myelopathy secondary to soft disc herniation exhibit the greatest gains in functional status following nonoperative care, probably due to the potential for spontaneous disc regression. Approximately 23% to 54% of patients who initially undergo conservative management convert to surgery due to failure to improve or disease progression. Furthermore, rates of hospitalization for subsequent SCI are higher in patients undergoing initial nonoperative treatment compared to those managed surgically.
Recent clinical practice guidelines have strongly recommended that patients with moderate and severe DCM undergo surgery in a timely fashion given the risk of further deterioration and lack of improvement with conservative management. There is still controversy about whether patients with mild myelopathy should undergo upfront surgical management or whether a trial of conservative treatment is reasonable. Are there clinical or imaging factors that can help make this decision? Are there genetic, CSF, serum or advanced imaging biomarkers that can detect subtle disease progression and point towards surgery as the optimal treatment strategy? And, finally, if nonoperative management is pursued, what should be the frequency and modality of therapy? How should we monitor these patients for disease progression and need for surgery? These questions reflect important knowledge gaps in the literature that must be answered by future research.
The non-operative management for patients with DCM should include close clinical follow-up to detect neurological deterioration as early as possible, patient education and symptom-directed therapy, e.g., medicinal pain therapy and physical therapy. However, there is currently insufficient evidence for specific therapy recommendations.
Optimizing rehabilitation after surgery
Rehabilitation after surgery is a mainstay of many conditions but perhaps underutilized in DCM; this is in contrast to acute SCI, for which rehabilitation is recommended in virtually all patients. In DCM, rehabilitation is often considered optional in patients with severe walking impairment. Importantly, the ability to walk without aids does not imply that patients with DCM do not have gait impairment. For example, it has been shown that patients with DCM have delayed voluntary and reactive motor commands, resulting in balance impairment [10]. More work is needed to integrate this knowledge into specific rehabilitation strategies for patients with DCM.
The value of multidisciplinary engagement to improve surgical outcomes
Research has shown that patients have improved surgical outcomes if they are younger, do not smoke or suffer from psychiatric disorders, have a shorter duration of symptoms, and have less severe myelopathy without gait impairment. Many of these patient characteristics are modifiable but require multidisciplinary input. For example, increasing awareness of DCM and developing diagnostic criteria will allow primary care physicians and other healthcare professionals to identify patients in a timely fashion (i.e., shorter duration of symptoms), at milder disease stages (i.e., less severe myelopathy), and before the development of gait impairment. Furthermore, non-surgical practitioners can aid with smoking cessation either with behavioral or pharmacological therapies and help provide appropriate management for psychiatric comorbidities. Finally, physical therapists and physiatrists can help to optimize a patient’s functional status prior to surgery and aid in rehabilitation post-operatively.
Conclusions
DCM is a preventable cause of non-traumatic SCI. The development of diagnostic criteria for DCM would benefit from the experiences of neurologists since many patients are initially diagnosed by them (11). Commonly known criteria will help the dialog between neurologists, primary care providers and spine specialists, and propagate the knowledge of red flags in DCM that require timely and specific actions, before established SCI.
The best care for patients with spinal cord disorders requires teamwork. Primary care physicians and allied health providers must carry a high degree of clinical suspicion when encountering individuals with recurrent falls or subtle neurological signs and symptoms that may localize to the cervical spine. Neurologists must be able to identify red flag signs and symptoms of DCM and refer early for neuroimaging and surgical evaluation. These providers are also essential for monitoring patients with DCM who are initially treated conservatively. Spine surgeons must identify ideal surgical candidates and prioritize individuals with moderate and severe disease to ensure timely decompression. Rehabilitation physicians and physical therapists must work together with patients to optimize preoperative functional status and promote recovery postoperatively.
About the authors
Dr Lindsay Tetreault’s research has focused on identifying important clinical and imaging predictors of outcomes and complications in patients undergoing surgery for DCM. She was also instrumental in developing clinical practice guidelines for the management of individuals with mild, moderate, and severe DCM as well as non-myelopathic patients with evidence of spinal cord compression. She is an active member of the AO Spine RECODE-DCM project and one of the leaders of the diagnostic criteria incubator. Lindsay hopes to develop valid and reliable criteria that can assist healthcare providers in making a diagnosis of DCM in a timely fashion.
Dr Carl M. Zipser, MD FEBN, is a board-certified neurologist and neurophysiologist who specializes in spinal cord disorders. He is currently working at the Spinal Cord Injury Centre at Balgrist University Hospital, Zurich, Switzerland. His work is committed to evaluating CSFP in patients with acute and chronic spinal cord compression. Furthermore, his research also focuses on advanced neurophysiology and neuroimaging of the spinal cord. He is a member of the steering committee of AO Spine RECODE-DCM and is actively involved in the diagnostic criteria incubator.
References and further reading
- Behrbalk E, Salame K, Regev GJ, Keynan O, Boszczyk B and Lidar Z. Delayed diagnosis of cervical spondylotic myelopathy by primary care physicians. Neurosurg Focus 2013;35(1):E1
- Smith SS, Stewart ME, Davies BM and Kotter MRN. The Prevalence of Asymptomatic and Symptomatic Spinal Cord Compression on Magnetic Resonance Imaging: A Systematic Review and Meta-analysis. Global Spine Journal 2021;11(4):597-607
- Zipser CM, Margetis K, Pedro KM, Curt A, Fehlings M, Sadler I, et al. Increasing awareness of degenerative cervical myelopathy: a preventative cause of non-traumatic spinal cord injury. Spinal Cord 2021; 59:1216-1218
- Hupp M, Pfender N, Vallotton K, Rosner J, Friedl S, Zipser CM, et al. The Restless Spinal Cord in Degenerative Cervical Myelopathy. AJNR American Journal of Neuroradiology 2021;42(3):597-609
- Jutzeler CR, Ulrich A, Huber B, Rosner J, Kramer JLK and Curt A. Improved Diagnosis of Cervical Spondylotic Myelopathy with Contact Heat Evoked Potentials. Journal of Neurotrauma 2017;34(12):2045-53
- Zipser C, Pfender N, Kheram N, Boraschi A, Aguirre J, Ulrich N, et al. Intraoperative monitoring of CSF pressure in patients with degenerative cervical myelopathy (COMP-CORD Study): a prospective cohort study. Journal of Neurotrauma 2021; 39:300-310
- Zipser CM, Spirig JM, Aguirre J, Hofer AS, Pfender N, Hupp M, et al. Safety and Feasibility of Lumbar Cerebrospinal Fluid Pressure and Intraspinal Pressure Studies in Cervical Stenosis: A Case Series. Acta Neurochirurgica Supplement 2021; 131:367-72
- Zipser CM, Pfender N, Spirig JM, Betz M, Aguirre J, Hupp M, et al. Study protocol for an observational study of cerebrospinal fluid pressure in patients with degenerative cervical myelopathy undergoing surgical deCOMPression of the spinal CORD: the COMP-CORD study. BMJ Open 2020;10(9):e037332
- Zipser C, Cragg J, Guest J, Fehlings M, Jutzeler C, Anderson A, et al. Cell-based and stem-cell-based therapies for spinal cord injury: evidence from clinical trials. The Lancet Neurology 2022;In Press
- Boerger TF, McGinn LR, Wang MC, Schmit BD and Hyngstrom A. Degenerative Cervical Myelopathy Delays Responses to Lateral Balance Perturbations Regardless of Predictability. J Neurophysiol 2022;127(3):673-688
- Hilton B, Tempest-Mitchell J, Davies B and Kotter M. Route to diagnosis of degenerative cervical myelopathy in a UK healthcare system: a retrospective cohort study. BMJ Open 2019;9(5):e027000
Read more about the AO Spine RECODE-DCM initiative.
Read Dr Ben Davies's guest article on diversity and innovation in spine surgery.
Read Dr Ayush Sharma's guest article on recent updates and future directions in degenerative cervical myelopathy.
Disclaimer
The articles included in the AO Spine Blog represent the opinion of individual authors exclusively and not necessarily the opinion of AO Spine or AO Foundation.