Symphony for complex C-spine revision surgery - a case report
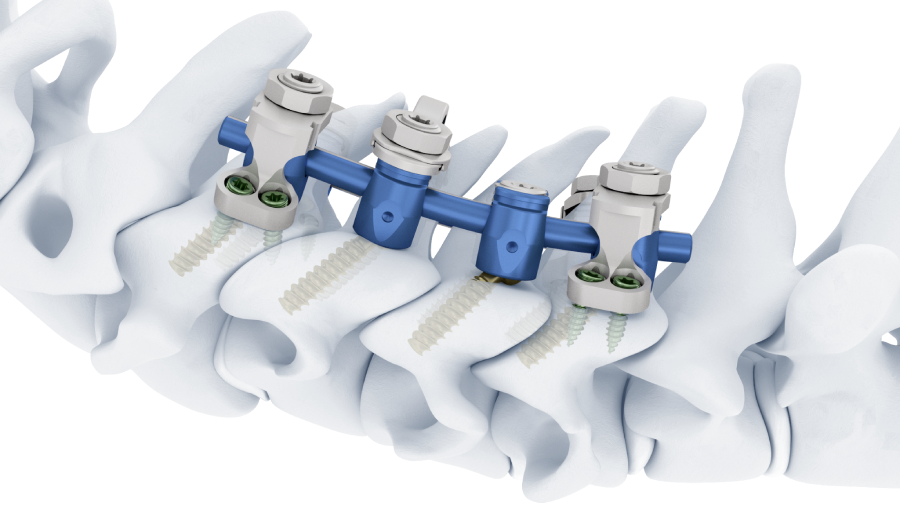
Launched in November 2019, the Symphony Occipito-Cervico-Thoracic System is an enhanced set of instruments and implants for posterior fixation of the upper (cervico-thoracic) spine. A full description of Symphony can be seen here.
Patient medical history
The woman was a 67-year-old internal medicine specialist who had six spine surgeries over the last 22 years. Beside her cervical spine problems, she was fit, healthy and slim. She first underwent cervical spine surgery in 1998 with a Frykholm procedure at C6/C7. Four years later she had another Frykholm procedure at C3/C4, followed shortly by an anterior cage stand-alone fusion procedure at C3/4. In 2015 she underwent a cage stand-alone anterior decompression and fusion at C5/C6 and C6/C7. Another 2 years later she had total disc prosthesis at C4/C5, and finally 16 months ago a posterior stabilization from C2 to C4.
Complaints
When the patient was seen for the first time in February 2020, she complained of significant load-dependent neck pain (Visual Analog Scale [VAS] score: 6 points) and an “awful” crepitation. She also reported bilateral arm pain (VAS score: 5 points) radiating to the ulnar side. She scored 30 points on the Neck Pain Disability Index. With neck flexion she experienced pain relief but also some tingling in her legs. The patient had tried to reduce the pain by conservative means including physiotherapy, massage, acupuncture, and multimodal pain treatment including continuous morphine therapy, but she did not improve substantially.
Clinical evaluation
Clinically, the patient had local pressure pain in the motion segments C4/5, C5/6, and especially C7/Th1 accompanied by a C8 radiculopathy on both sides. The slim neck and the muscle atrophy allowed an easy palpation of the posterior implants that was painful on the right side. Neurological and neurophysiological evaluation confirmed C8 root compression and residual myelopathic changes with prolonged Medianus-SEPs and MEPs. The ear, nose, and throat evaluation were within the reference range.
Radiographic evaluation
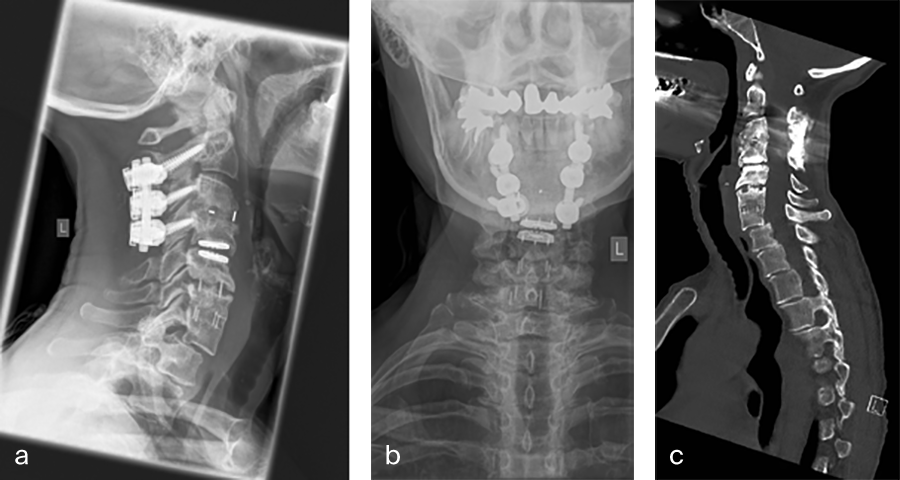
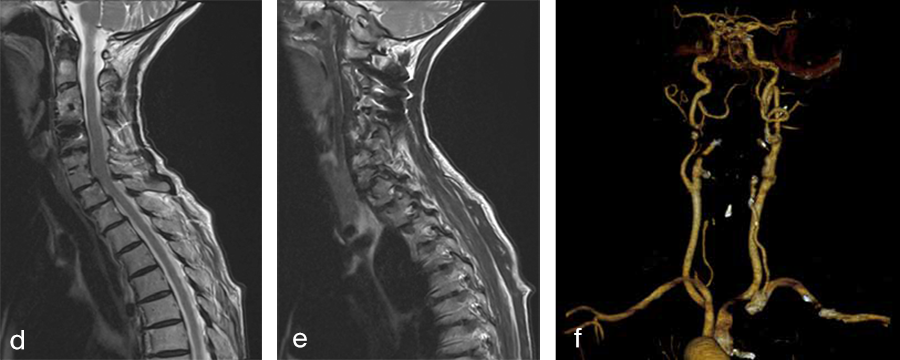
Her preoperative radiographic evaluation comprised two standard plane radiographs, functional radiographs, computed tomographic and magnetic resonance imaging scans (Fig 2) demonstrated:
- A fusion of the motion segments C2/3/4 and C6/7
- A moderate implant loosening of the posterior C2 screw on the right side
- An afunctional disc prosthesis at the level C4/C5 with significant heterotopic ossifications (grade III) accompanied by a facet joint osteoarthritis in this motion segment
- A nonunion at the level C5/C6 with residual mobility in this motion segment
- A highly mobile degenerative spondylolisthesis at the level C7/Th1 with bilateral neuroforaminal stenosis
Fig 2a–f Preoperative imaging showing: C2-C4 posterior instrumentation and fusion, anterior fusion after anterior cervical decompression and fusion (ACDF) C3/4 and C6/7, status after total disc replacement of C4/5 with heterotopic ossifications, nonunion C5/6 after ACDF C5/6, spondylolisthesis C7/Th1 with neuroforaminal stenosis on both sides and a normal cervical angiogram (cave: artefacts).
Surgery
Based on the findings detailed above and the patient's excruciating pain, an anterioposterior revision surgery was performed in June 2020.
First, a C7/Th1 anterior cervical decompression and fusion was performed via a left-sided approach. After decompression of the spinal canal and bilateral neuroforaminal decompression of the C8 nerve route, stabilization was achieved with a stand-alone Syncage-C (DPS) that already significantly reduced the spondylolisthesis.
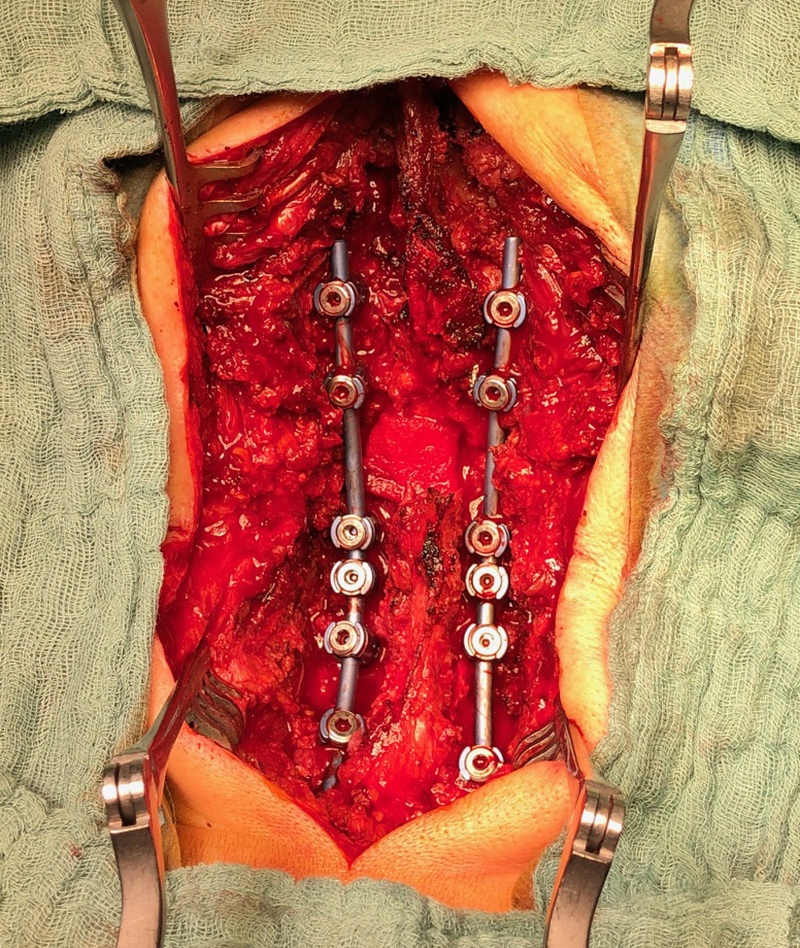
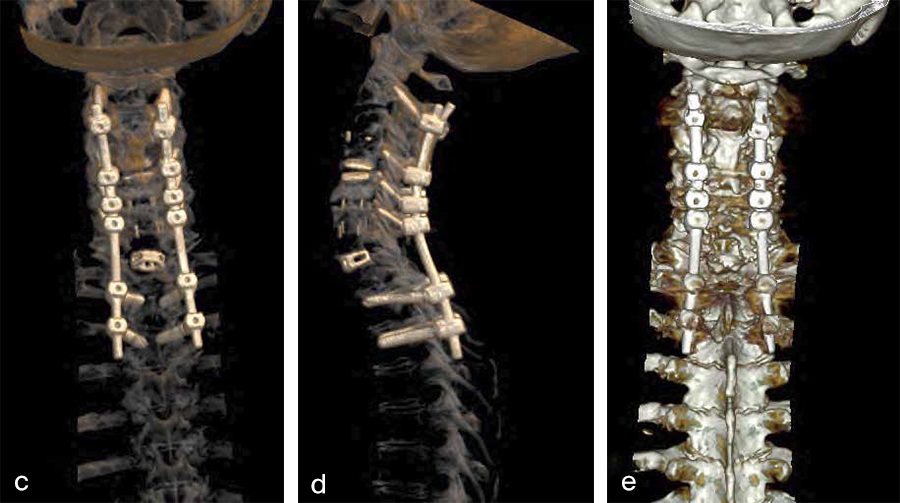
Second, a posterior revision surgery was performed including implant removal of the posterior instrumentation between C2 and C4 followed by a laminectomy of C7 with bilateral foraminotomy of the C8 nerve routes. Re-instrumentation with isthmic screws at the level C2, lateral mass screws at the level C4, C5, and C6, as well as bilateral pedicle screws at the level T2 and T3 were performed using the new Symphony system (DPS). The Symphony system offered the opportunity to place 4.0 mm screws in the previous loosened screw location at the level C2. The 3.5 mm screws were placed in C4, C5, and C6, and 5.5 and 5.0 mm screws at T2 and T3, respectively. The 4.0 mm rod allowed a good direct connection between the cervical spine isthmic and lateral mass screws and the thoracic pedicle screws, providing adequate stability and allowing excellent reduction. An intraoperative image is shown in Fig 3. Postoperative radiographic evaluations are displayed in Fig 4.
Postoperative course
The patient improved significantly postoperatively regarding her C8 radiculopathy, which disappeared the day after surgery. Her neck pain also improved significantly and on the day of discharge from the hospital (day 5 after surgery) she only needed analgesic medication (administration of 600 mg ibuprofen three times daily).
At 6-week postoperative follow-up, the patient was taking 600 mg ibuprofen once daily for a “good night's sleep” and she was satisfied with the postoperative course (VAS neck pain score: 2; arm pain score: 0). Hence, her rehabilitation program was started.
Disclaimer:
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
The brands and labels "approved by AO Technical Commission" and "approved by AO Foundation", particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.