Viper Prime System
Pujan Kavakebi, Andreas Korge, Christian Mazel, Emiliano Vialle
Viper Prime is the next generation system for minimally invasive pedicle screw and rod placement from a posterior approach. It allows the surgeon to provide immobilization and stabilization of spinal segments as an adjunct to fusion in the treatment of acute and chronic instabilities or deformities of the thoracic, lumbar, and sacral spine.
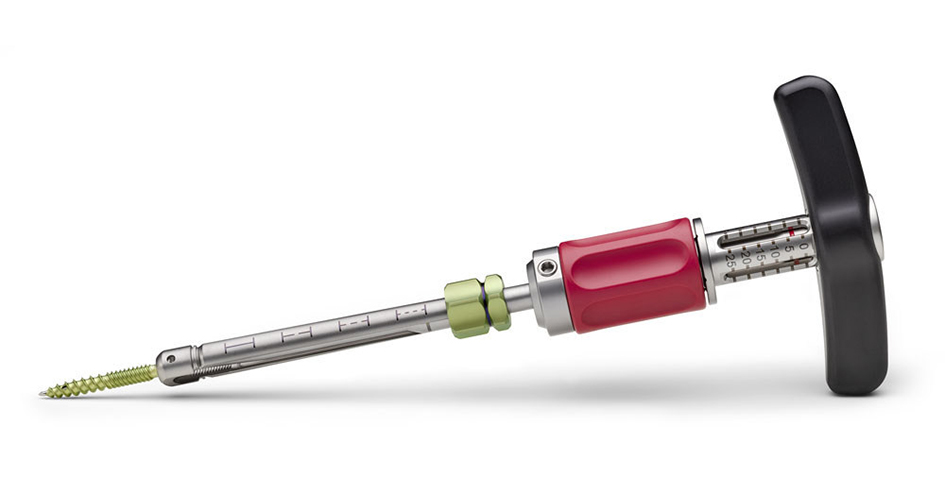
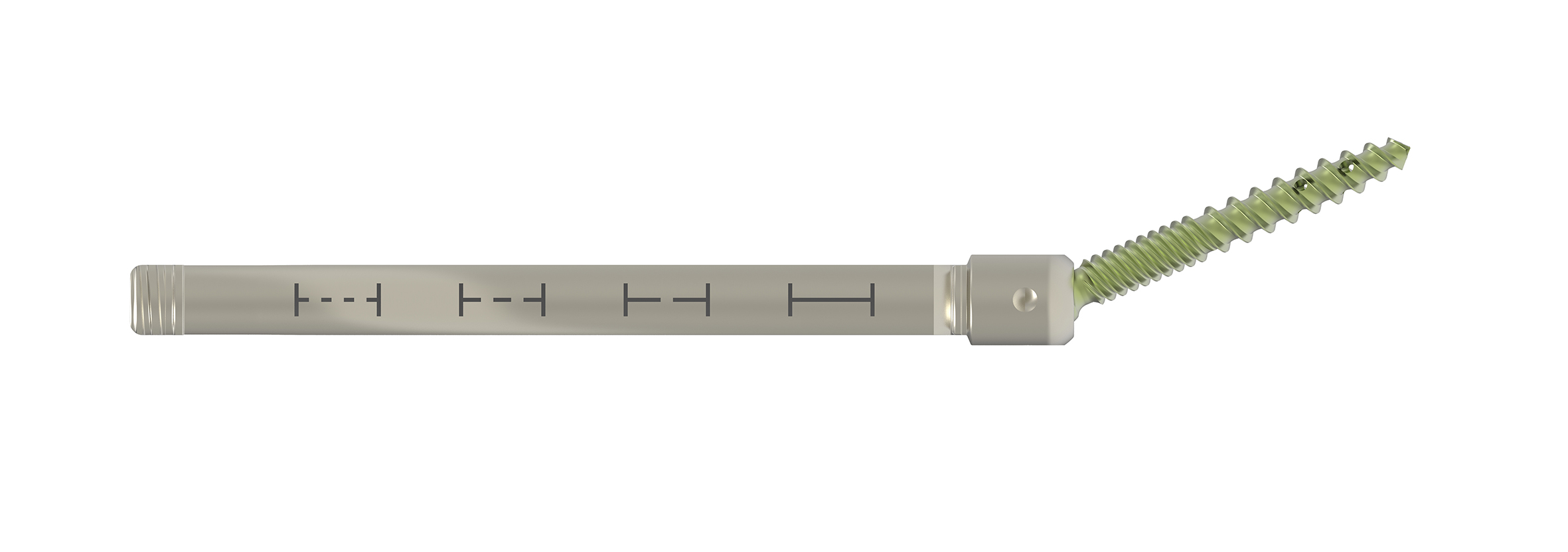
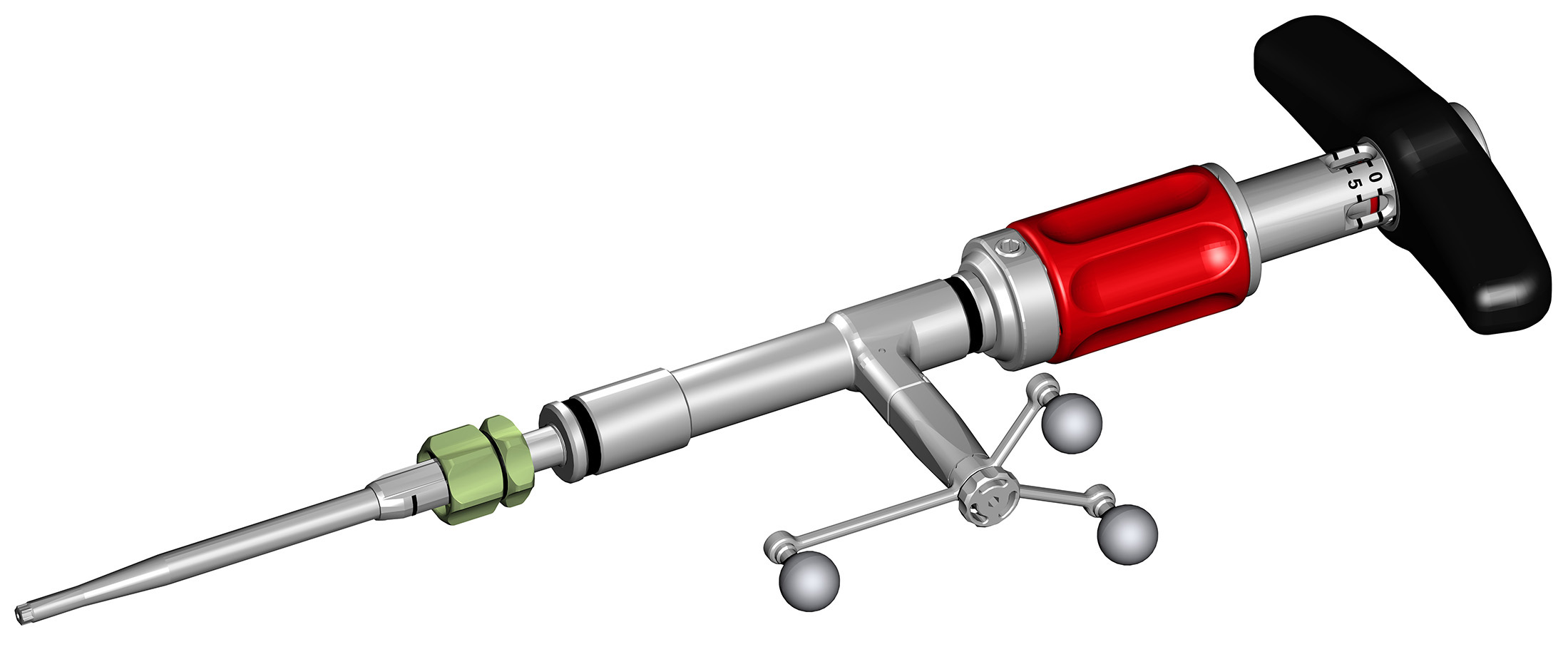
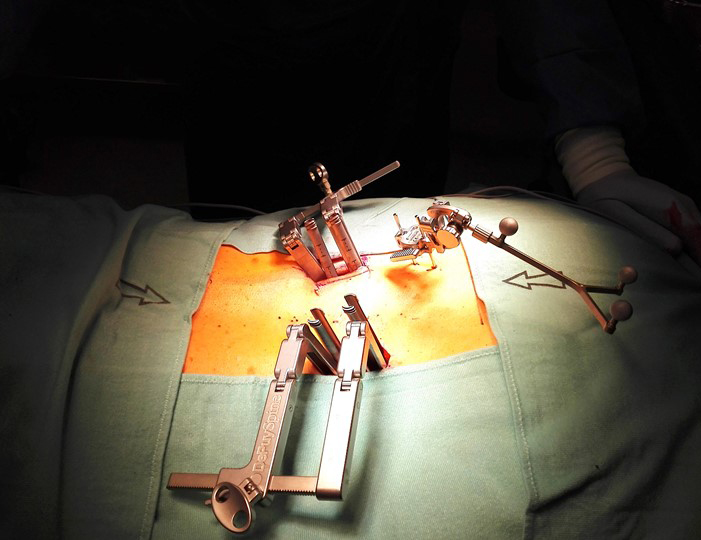
The Viper Prime system introduces a novel technique for percutaneous pedicle screw placement that eliminates the need for Jamshidi needles, guide wires, and pedicle preparation instruments. Utilizing an innovative self-starting screw tip design and a stylet (guide wire) that is fully controlled by the screwdriver, surgeons can target pedicles and insert the screw with one single instrument (Fig 1).
The surgeon is therefore able to realize the benefits of minimally invasive surgery without the need for guide wire management and complex instrument systems.
Background
The last resort and ultimate treatment option for low back pain is fusion surgery, which is performed worldwide in more than 1 million cases per year. Acceptable clinical results are achieved in only 65% to 75% of cases, which emphasizes the need for improvement in this surgical procedure. In one of seven cases there is an approach related problem, which explains the demand for optimizing the surgical approach and technique.
Minimally invasive techniques to perform spinal stabilization have gained popularity in recent years due to the demonstration of reduced complications, less blood loss, and less perioperative muscular damage during the procedure compared to conventional open spine surgery. Additionally, minimally invasive approaches yield less postoperative pain, less narcotic usage, a shorter hospitalization, and quicker recovery than traditional open surgery.
Whilst existing minimally invasive systems (eg, Expedium Spine system, Viper 2 system) offer substantial benefits, there are still some procedure-related disadvantages. Surgeons are often required to navigate small channels involving several procedural steps, instrument passes, and management of guide wires. To streamline the surgical procedure, the Lumbar Degenerative Expert Group (LDEG), together with DePuy Synthes, have developed the new Viper Prime system as the next generation system for minimally invasive pedicle screw and rod placement from a posterior approach.
Innovative design features of Viper Prime
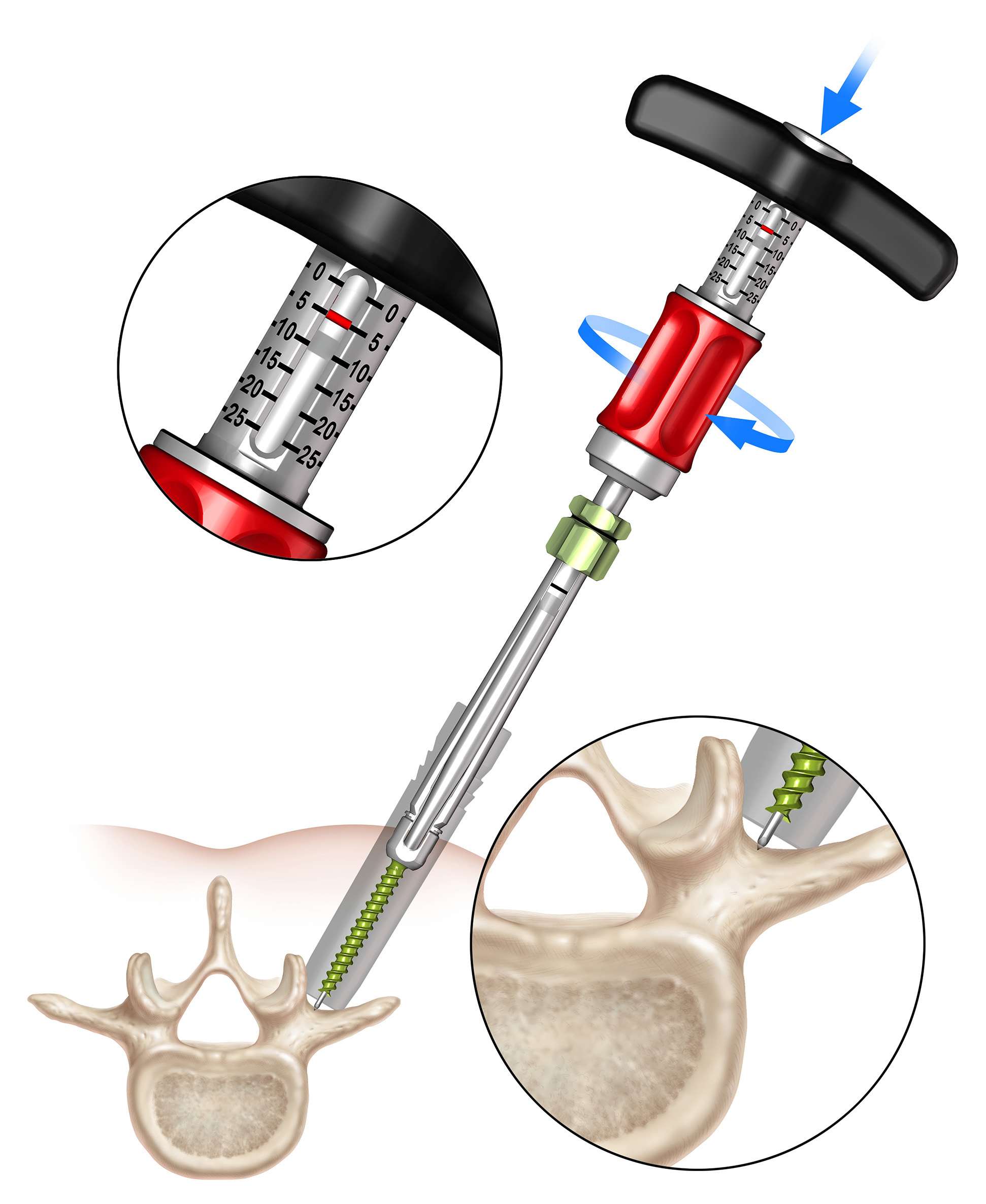
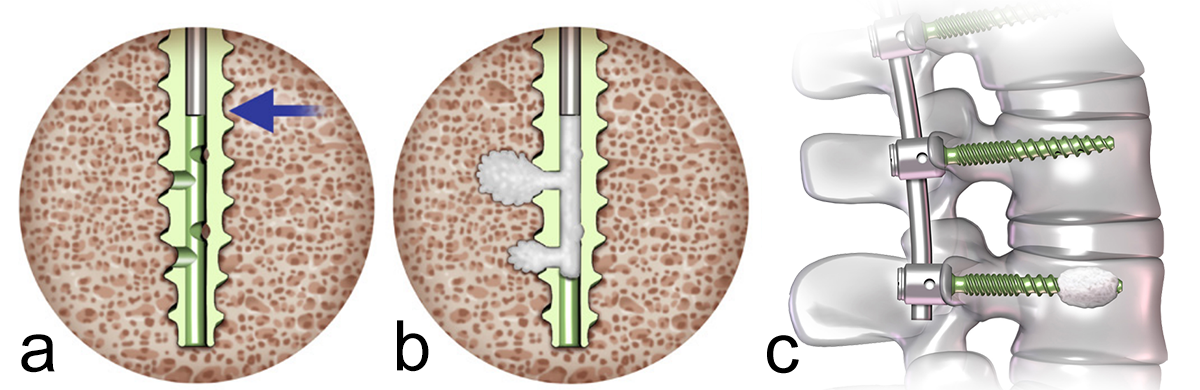
In the fully retracted position the stylet extends 3 mm beyond the tip of the screw, which allows proper docking to the vertebral body (Fig 2). The stylet is advanced by turning the red stylet control handle clockwise. The extension of the stylet relative to the screw tip is displayed by the red line visible through the drive tube window on the stylet depth gauge. There is also an audible click every 1 mm of extension.
Since the stylet is controlled by the inserter, this technique is safer and requires less radiation as compared to traditional techniques where Jamshidi/guide wire positions need to be established freehand and to always be checked by fluoroscopy.
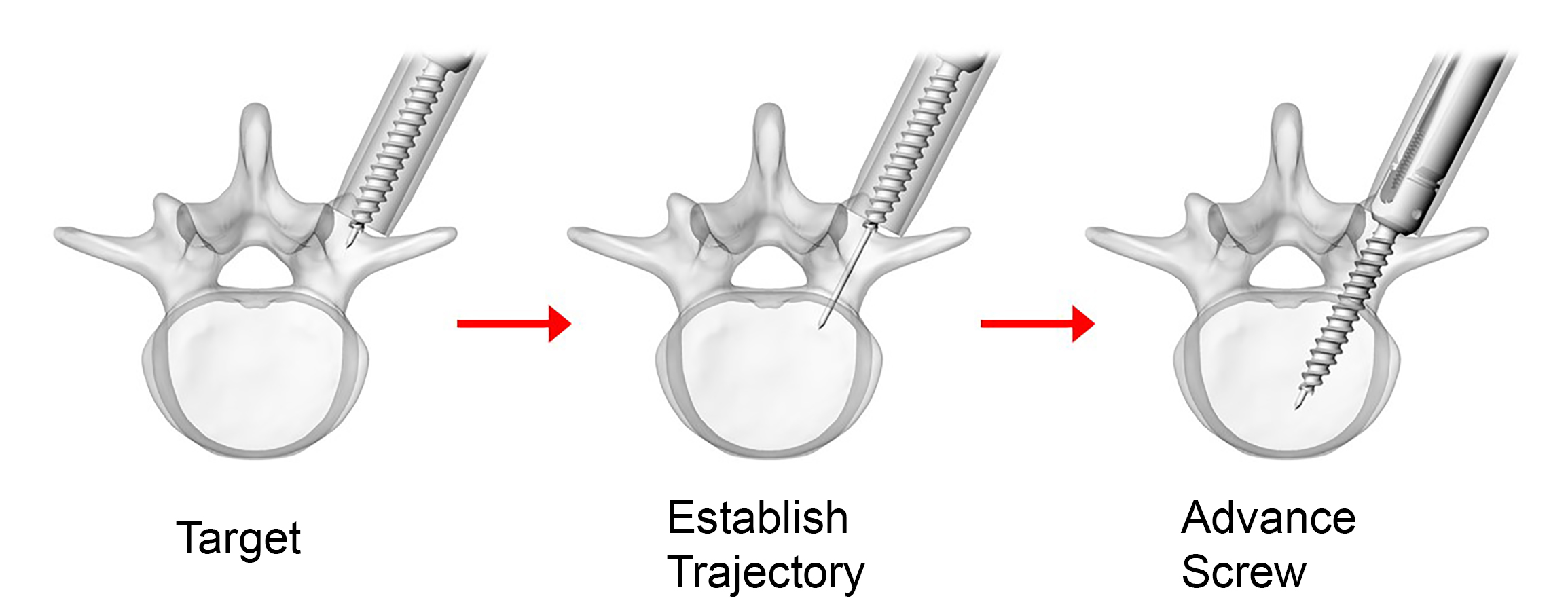
The surgeon uses the Viper Prime inserter for targeting, establishing the trajectory with the stylet, and advancing the screw in one single procedure (Fig 3). The stylet can be extended up to 25 mm to define the correct transpedicular corridor.
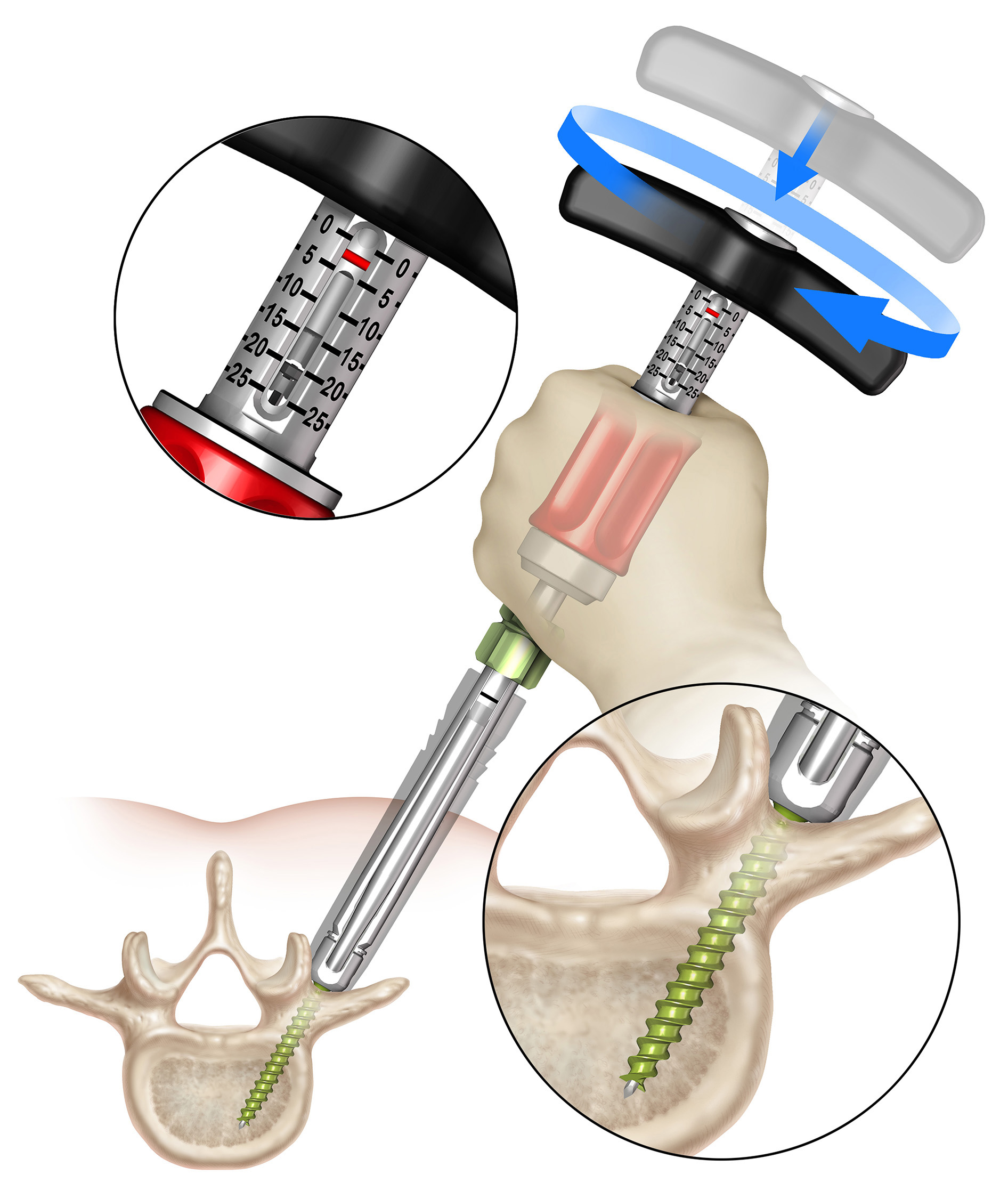
Once the stylet has been extended to establish the trajectory, the surgeon must hold the red stylet control handle while rotating the proximal modular handle (palm handle or T-handle) of the inserter clockwise to advance the screw into the pedicle over the extended stylet (Fig 4). It is critical to always hold the red stylet control while advancing the screw. Holding the red stylet control handle will retract the stylet as the screw is advanced into the pedicle. If the red stylet control handle is not held, the stylet will remain extended and advance in front of the screw, potentially leading to an anterior wall breach, neurological damage, or damage to the great vessels.
In addition to the standard screw the Viper Prime system also offers a cortical fix fenestrated screw (Fig 5). This screw has two different thread shapes (quad lead thread and dual lead thread) designed to optimize the purchase in the pedicle as well as in the vertebral body. The fenestration allows for the option of cement delivery (with CONFIDENCE High Viscosity Spinal Cement) for augmented screw fixation in patients with poor bone quality (eg, osteoporosis).
Viper Prime's reduced number of procedural steps and instruments improves the quality of life for the surgeon and nurses. All the instrumentation fits neatly into a 1-level case to minimize the amount of product on the back table in the OR.
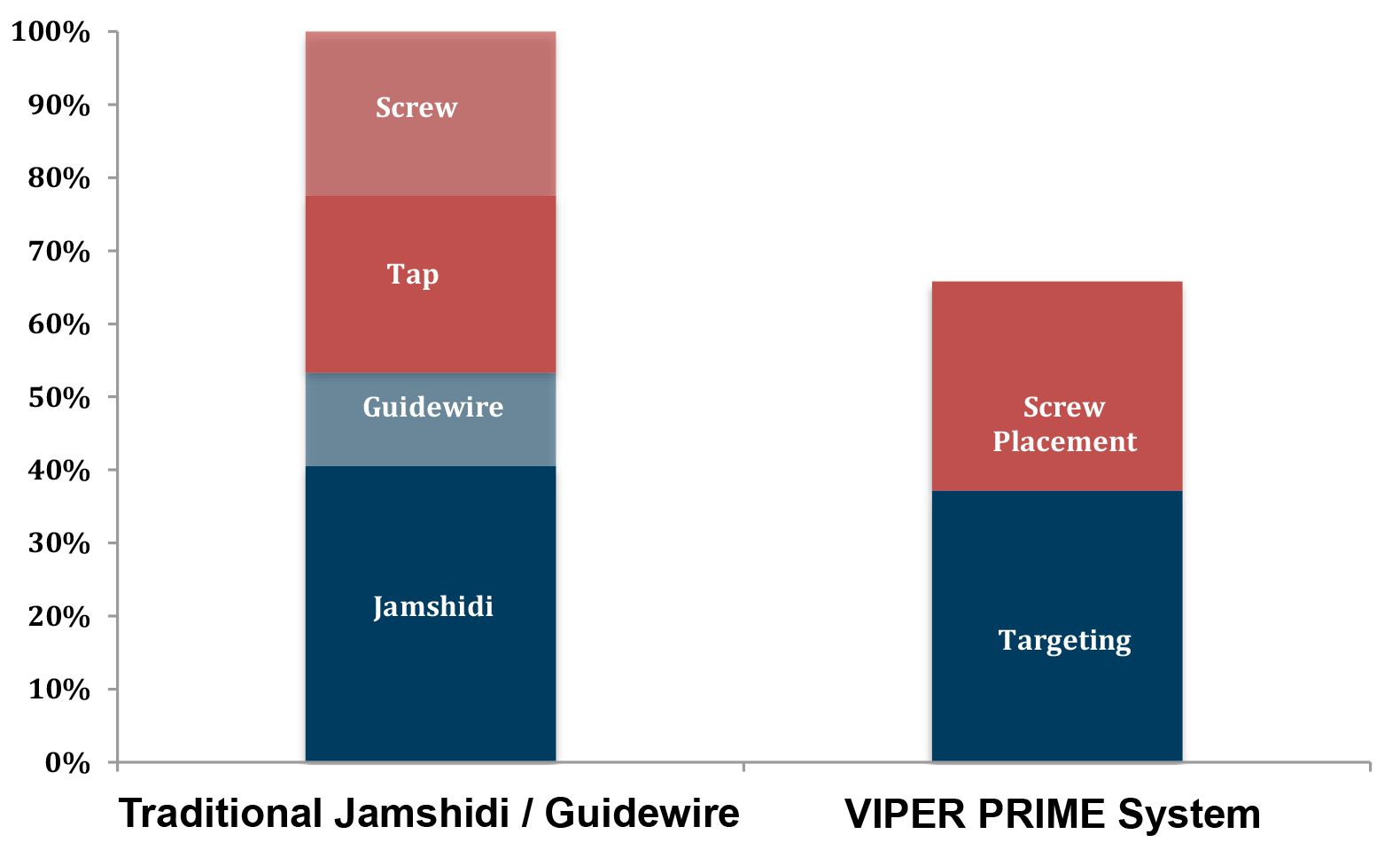
The new insertion technique reduces the time to complete screw insertion compared to traditional minimally invasive techniques by about 33%. This was shown by a cadaveric evidence generation study conducted by 8 surgeons across 7 levels each (Fig 6).
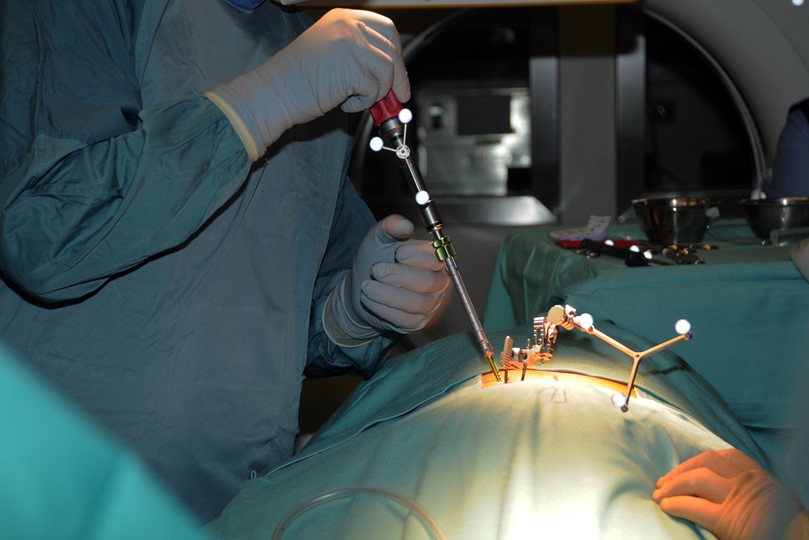
The instrumentation is available to accommodate both a standard fluoroscopic MIS procedure along with a navigated inserter (Fig 7), which is compatible with the Brainlab, Stealth, and Stryker navigation systems. In the navigated version, due to safety reasons (no fluoroscopic control) the stylet only comes out by 5 mm.
Case provided by Pujan Kavakebi, Innsbruck, Austria
Case: 52-year-old with low back pain
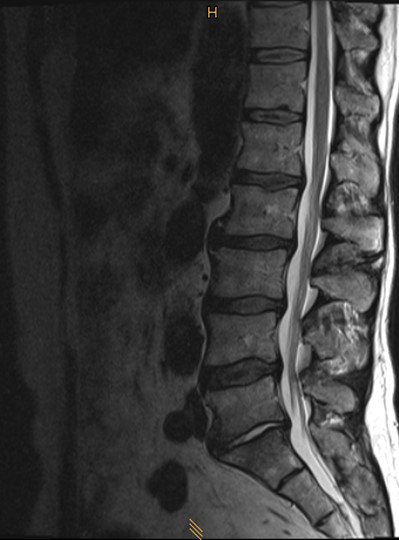
A 52-year-old male patient presented with low back pain and S1 radicular pain on the left side for 3 weeks. The laboratory tests of the blood showed enhanced inflammation signs. The MRI showed an infection of the L5/S1 disc (Fig 1). Indications for minimally invasive spondylodesis with intervertebral cage (Figs 2-6).
Overview of MIS pedicle screw insertion and Viper Prime technique
Safety and complications associated with cement-augmented pedicle screws―retrospective milestone D report
Ann-Sophie Neuts, MD, Maarten Spruit, MD
Introduction and clinical need
With an aging population there is increasing complexity in the treatment of various spinal problems which demand an operative solution. There is a decrease in bone quality resulting in osteopenia and osteoporosis. This may create associated and expected problems of screw pull out, screw loosening, and proximal junctional kyphosis (PJK) or failure (PJF). Preoperative workup and assessment of bone quality is mandatory [1]. This was recently also addressed by the AO Technical Commission Spine in an Osteoporotic Spine Surgery Task Force and recommendations for preoperative workup in elective spine surgery in patients older than 50 years were presented in a white paper report. Still, prevention of PJK and PJF is an ongoing debate for which the ultimate solution has not yet been found. The aim is always to address sagittal alignment and apply secure instrumentation as well as possible. Longer fusion trajectories seem to increase the risk of failure.
What we know is that cement-augmented pedicle screws (CAPS) can be used to provide additional vertebral bone purchase and fixation. A pedicle screw with good purchase will be a pedicle screw that can correct deformity, reduce slip, and maintain vertebral fixation until solid fusion occurs. In the spinal community there is a consensus that augmented pedicle screws are a good alternative in patients with poor bone quality and provide supplementary anchorage compared with traditional nonaugmented pedicle screws (Fig 1).
The polymethylmethacrylate (PMMA) cement which is used, such as CONFIDENCE™ High Viscosity Spinal Cement (DePuy Synthes), has excellent adhesive properties, augments the screw-bone interface to prevent screw pull-out, and therefore reduces the risk of screw loosening or failure. CAPS are used both in primary cases and in revision surgery.
The use of CAPS may also be associated with certain risks and potential complications. Cement leakage is one of the most common complications related to cement augmentation. The cement can extravasate into the surrounding tissues, including not intended entry into the spinal canal, and hence compromise the spinal cord, cauda and nerve roots, or damage/invade blood vessels. This can result in radiculopathy, neural tissue compression, and even vascular compromise and pulmonary embolism. The incidence of cement leakage varies from 6% to 43%, but fortunately leakage is symptomatic in only 0.6− 1.9% [2, 3]. During the exotherm-curing process of cement, heat is generated. The heat can cause thermal injury to the surrounding tissues, resulting in neurological deficits, such as sensory or motor dysfunction, radicular pain, and bladder or bowel dysfunction. The risk of thermal injury increases with the use of excessive cement volume [3, 4].
Although cement augmentation enhances screw fixation, there is still a risk of screw loosening, screw migration (2.2%), or screw breakage (0.6%) due to mechanical failure caused by cyclic loading and stress [3, 5]. As with any surgical procedure there is also a risk of infection. The presence of cement may complicate the management of infection due to a potential heightened risk of biofilm formation. Infection incidence is estimated to be 1.1−5.7% [3, 6].
Material and results
For this Milestone D report, we reviewed 61 patients who had cement-augmented pedicle screw spine surgery between July 2020 and March 2023. In our hospital we use Expedium Verse Advanced Fenestrated Cortical Fix Polyaxial Screws® (DePuy Synthes). The group included 9 men and 52 women with an average age of 62.5 years (range: 31−83 years). 19 (31%) of the 61 operations were revisions of earlier constructs due to hardware failure or PJK/PJF. The other 42 cases were primary fusions with the need to use cement-augmented screws because of poor bone quality, osteopenia, or osteoporosis. Table 1 shows the trajectory of fusion in primary and revision surgery. Most cases included five or more fusion levels. In 61 patients we used a total of 464 fenestrated screws. Cement augmentation was used in 233 screws.
| Primary Surgery | Revision Surgery | |
| 1 Level | 5 | 3 |
| 2 Level | 4 | 4 |
| 3 Level | 2 | 2 |
| 4 Level | 5 | 2 |
| ≥ 5 Level | 26 | 8 |
Table 1: Trajectory of fusion in primary and revision cases.
Cement leakage
We had four cases (four screws) with cement leakage (1.7%). One patient had an anterior vertebral cortical breach due to placement of the screw. Another sustained a lateral breach of the pedicle wall with leakage into the transverse process. In both patients, leakage was noticed during surgery as we always augment each pedicle screw under image intensifier control. Augmentation was ceased as the moment leakage was observed.
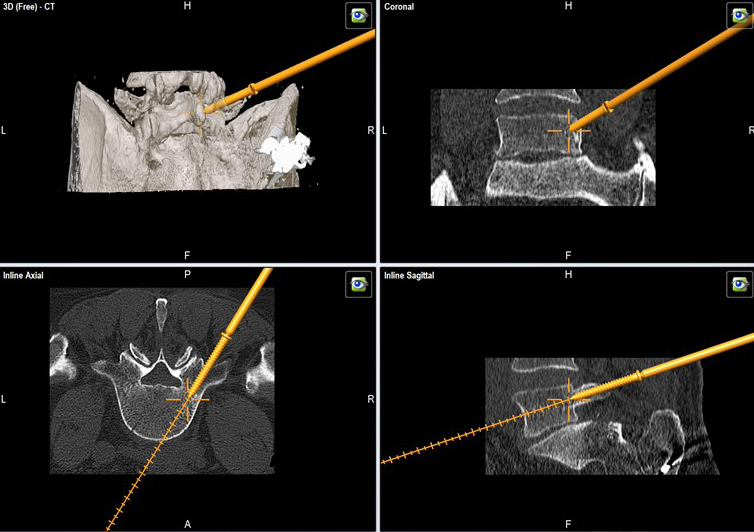
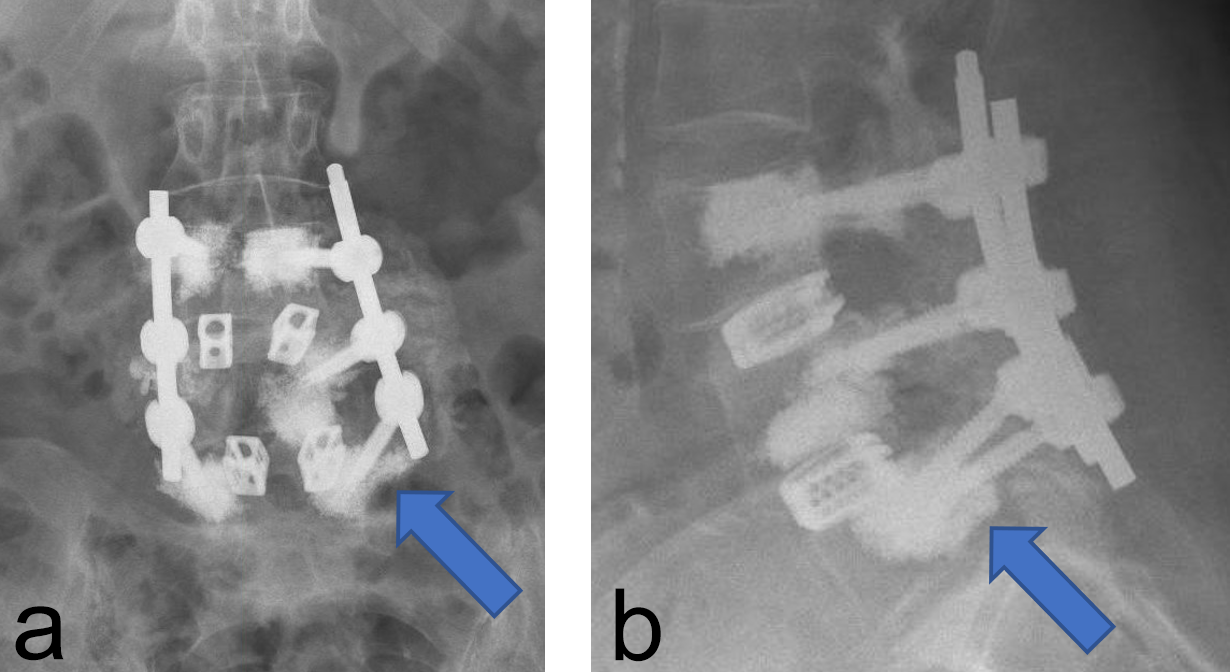
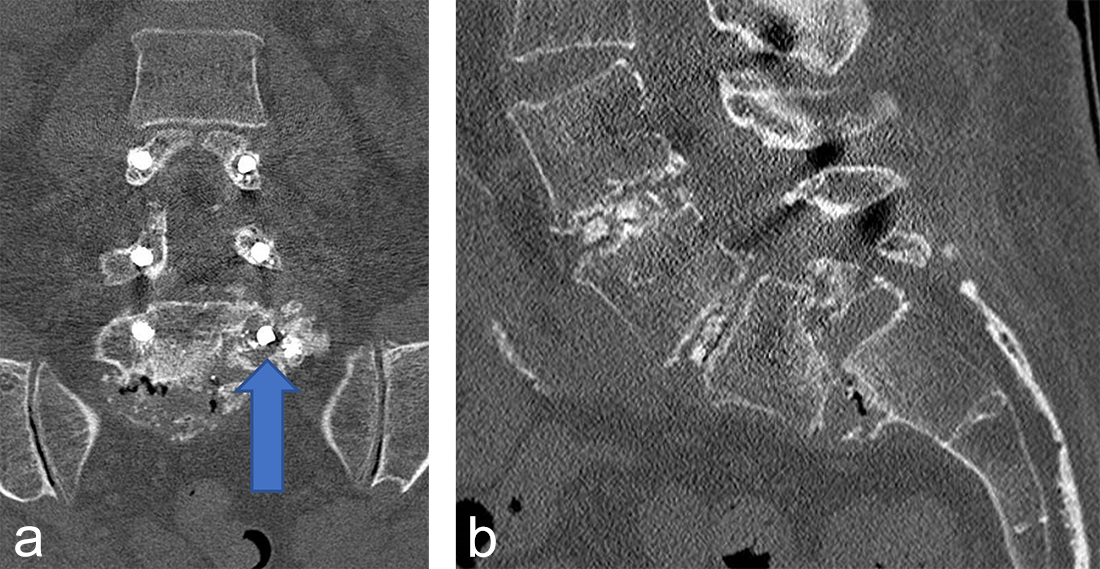
Two other patients had cement leakage into the neuroforamen which caused motor and sensory dysfunction of the affected nerve root. In retrospect the chosen screw length was too short for the vertebra; therefore, the most posterior located perforation in the screw was located within the pedicle (Fig 2).
Other complications
Six (9%) of 61 patients developed a surgical site infection. All had a debridement, antibiotics, and implant retention in the operating room and antibiotic therapy for 3 months. There is extensive variability in literature on the incidence of infection after instrumented spine surgery [7]. Zhou et al [7] described an incidence of 6% in degenerative cases (noninstrumented and instrumented combined). The infection risk is higher in instrumented surgery and the risk increases with prolonged surgery and higher amount of perioperative blood loss. Still, 9% infection rate in our series is relatively high, especially compared with our overall institutional spinal infection rate of 2%. This may be due to multiple revisions in these 6 patients (50%) and the long fusion trajectory with large exposure and longer duration of surgery (33%).
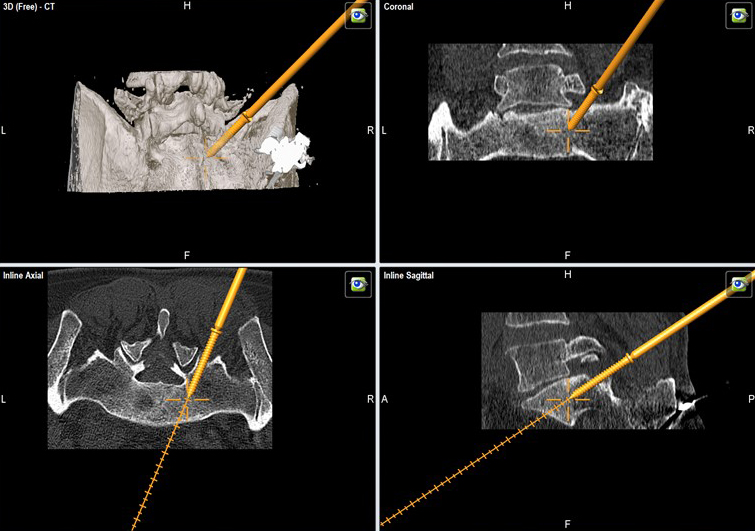
Instrumentation failure occurred in 7 (11.5%) of 61 patients. One patient developed loosening of the three most caudal nonaugmented screws in a long fusion construct. Two patients had screw loosening after a proven infection (one of them with augmented screws). One developed screw loosening due to a pedicle fracture. Another had CAPS loosening after traumatic fracture of the L5 vertebral body (Fig 3). Two patients had a screw breakage. In total 3 (0.86%) of 233 cement- augmented pedicle screws in 61 patients failed despite cement augmentation (Table 2).
| Nonaugmented PS* | CAPS* | |
| Screw loosening | 1 | 0 |
| Infection + screw loosening | 1 | 1 |
| Pedicle No. + screw loosening | 1 | 0 |
| Screw breakage | 1 | 1 |
| Vertebral No. + screw loosening | 0 | 1 |
Table 2: Cases of instrumentation failure in nonaugmented and augmented screws. *PS indicates pedicle screw; CAPS, cement-augmented pedicle screws.
Clinical case
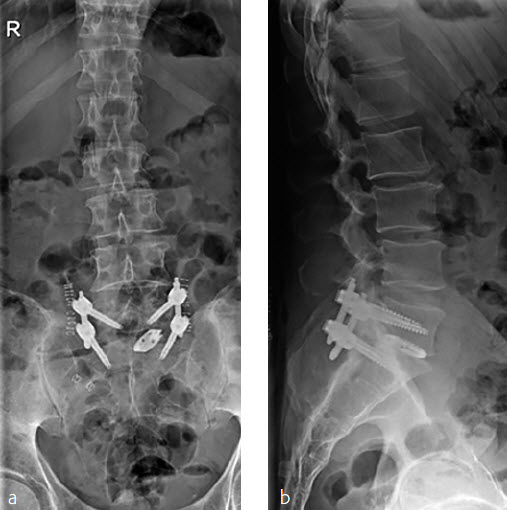
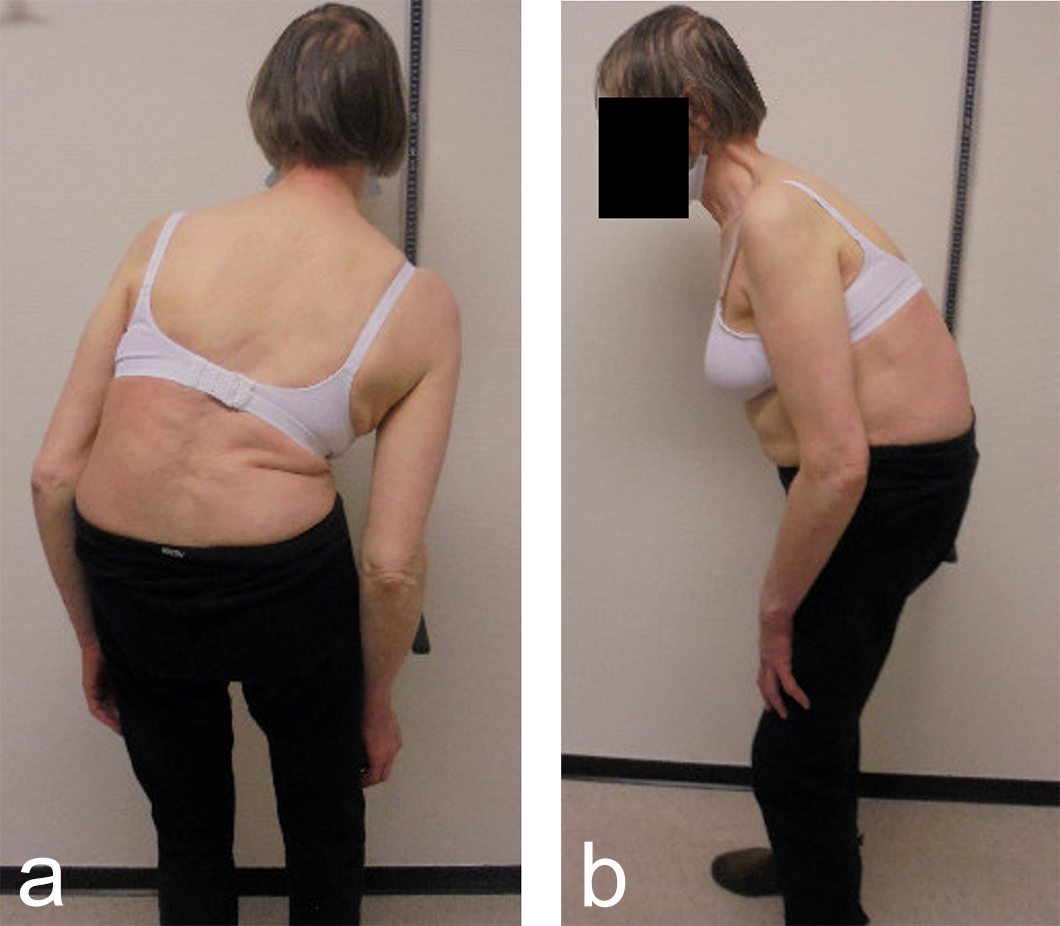
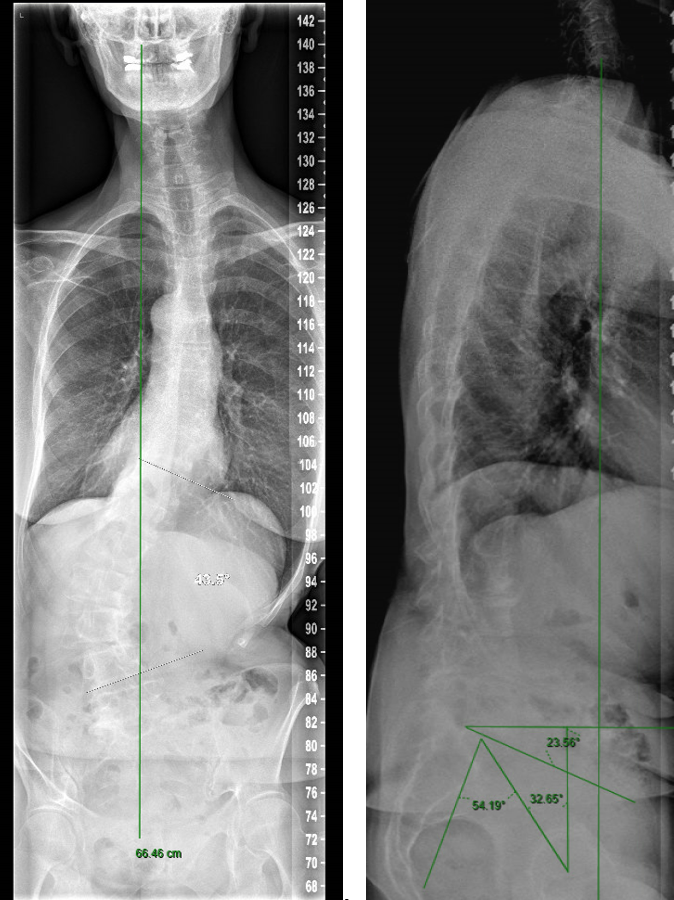
A 70-year-old woman with an adult spinal deformity (ASD) was referred to our hospital for a second opinion. Her medical history included rheumatoid arthritis for which she was given long-term methotrexate therapy and cures of corticosteroids. She presented with a severe degenerative deformity and right L3 radiculopathy. The patient was completely off balance with a right coronal shift and a positive sagittal balance with a SVA of 10 cm+ (Fig 4). Figure 5 reveals full spine images and pelvic parameters. She led a sedentary lifestyle because of the disturbance of balance and back and right leg pain. The patient opted for surgery after a shared decision process and informed consent.
The surgery was planned as a posterior approach to correct the spinal deformity and restore coronal as well as sagittal balance with direct and indirect decompression of the right L3 nerve root. The preoperative bone assessment and DEXA scan indicated osteopenia and considering her medical history the decision was to use cortical fix screws that also allowed cement augmentation.
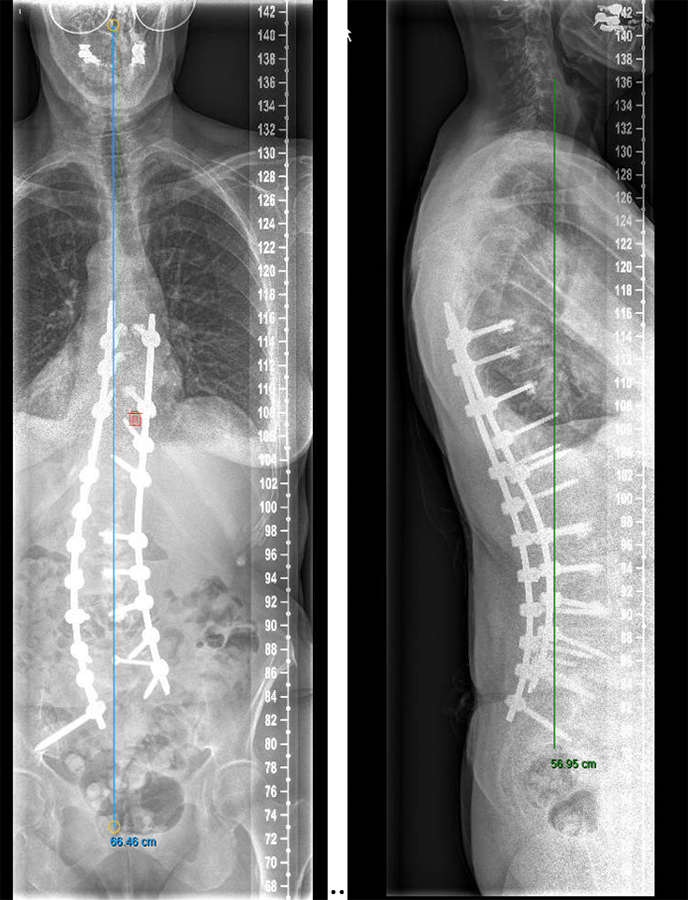
We performed a posterior fusion and correction of Th8-S2-SI-Ilium (Fig 6). In Th9, Th10, Th11, and Th12 we instrumented only unilateral pedicle screws due to small pedicle diameters. A S2-SI-Ilium screw was placed on the left side. A supplemental translaminar screw was used at L5-S1 on the right side. We augmented the screws in Th8, Th9, Th10, L2, L3, and L4. Additionally, a TLIF approach was performed on L2-3 and L3-4 with autogenous bone as interbody support. No complications occurred during nor after surgery, and she remained in good balance during the 1-year postoperative follow-up.
Conclusion
Cement-augmented pedicle screws are effective and safe to use in patients with poor bone quality who require spinal instrumentation. Accurate surgical technique and safe cement augmentation under image intensifier guidance is mandatory. Cement leakage in this Milestone D report is 1.7%, which is low compared with published data. Cement-augmented screw failure is 0.86%. Other complications are not related to this type of instrumentation but to the surgical procedure itself.
References
- Sardar ZM, Kim Y, Lafage V, et al. State of the art: proximal junctional kyphosis diagnosis, management, and prevention. Spine Deform. 2021 May;9(3):635−644.
- Lieberman IH, Hardenbrook MA, Wang JC, et al. Assessment of pedicle screw placement accuracy, procedure time, and radiation exposure using a miniature robotic guidance system. J Spinal Disord Tech. 2012;25(5):241−248.
- Zhang J, Wang G, Zhang N. A meta-analysis of complications associated with the use of cement-augmented pedicle screws in osteoporosis of spine. Orthop Traumatol Surg Res. 2021 Nov;107(7):102791.
- Ledonio CG, Polly DW Jr, Swiontkowski MF. Management of thoracolumbar spine fractures. Spine J. 2014;14(1):145−164.
- Kim CW, Lee YP, Taylor W, et al. Use of polymethylmethacrylate-augmented pedicle screws for the surgical treatment of a thoracolumbar burst fracture: case report. J Neurosurg Spine. 2008;8(3):287−291.
- Lertudomphonwanit T, Goel VK, Traynelis VC, et al. Biomechanical effect of bone cement augmentation on pedicle screw fixation in different quality of bone: a finite element analysis. Spine (Phila Pa 1976). 2016;41(6):E323−E328.
- Zhou J, Wang R, Huo X, et al. Incidence of surgical site infection after spine surgery: a systematic review and meta-analysis. Spine (Phila Pa 1976). 2020 Feb 1;45(3):208−216.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.