CONCORDE Clear MIS Discectomy Device
Christian Mazel, Emiliano Vialle
The newly developed CONCORDE Clear MIS Discectomy Device was designed for more efficient disc clearing and endplate preparation in less time than using standard discectomy tools. As such, it simplifies discectomy in minimally invasive spinal fusion surgery. As it collects disc material, surgeons are able to quantify and estimate the volume removed during the procedure.
Minimally invasive surgery (MIS) is the fastest growing field in spinal fusion surgery because it has been shown to result in fewer complications compared to open surgery in treating degenerative disc disease. As this procedure becomes more popular, there is a high clinical demand to simplify MIS techniques and to develop new instruments that can improve the efficiency of the procedure.
Interbody fusion is one of the gold standard procedures in the treatment of lumbar degenerative disc disease. It is estimated that 140,000 discectomies concomitant to interbody fusion procedures will be performed across Europe, Middle-East, and Africa in 2018, of which a growing number will be minimally invasive.
Discectomy in spinal fusion surgery typically requires the surgeon to work through a small surgical field with limited visibility. Traditionally, it requires several instruments and multiple instrument passes to make sure that the disc is cleared, and that the endplates of the vertebrae are prepped for implants. Multiple passes increase the risk of nerve root injuries.
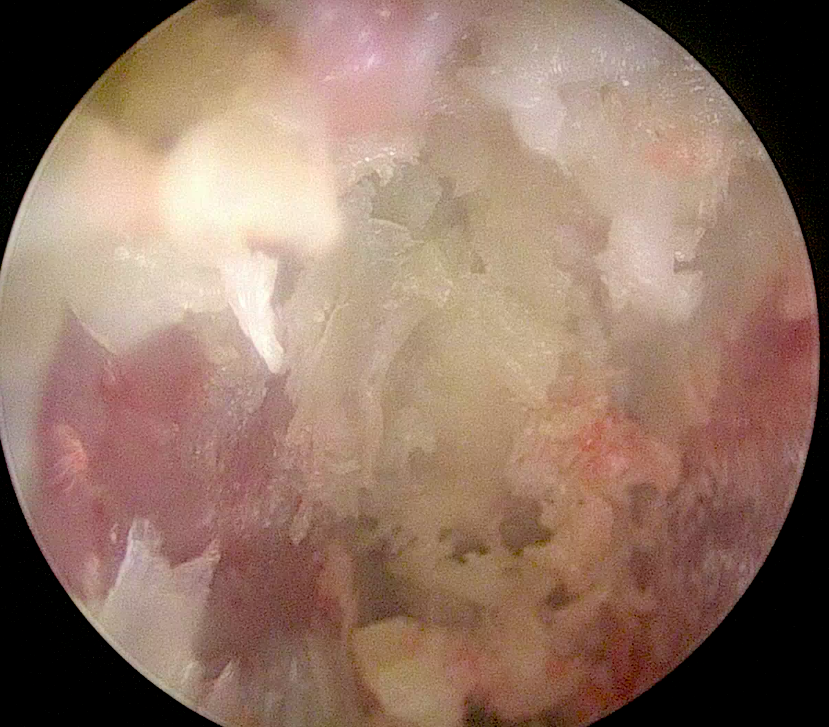
Although surgeons assume that posterior discectomy performed with traditional tools can adequately prepare the vertebral endplates for fusion, cadaveric studies have shown that an aggressive discectomy usually removes no more than 60% of the disc material [1]. In a recent cadaver course, participants were asked to work consecutively 20 minutes on the disc removal through a tubular portal. An endoscope control was then performed for direct visualization of the endplates. To the surprise of the participants, they had to admit that the disc preparation was not as good as they had anticipated. They all agreed that complementary discectomy and endplate preparation was needed (Fig 1).
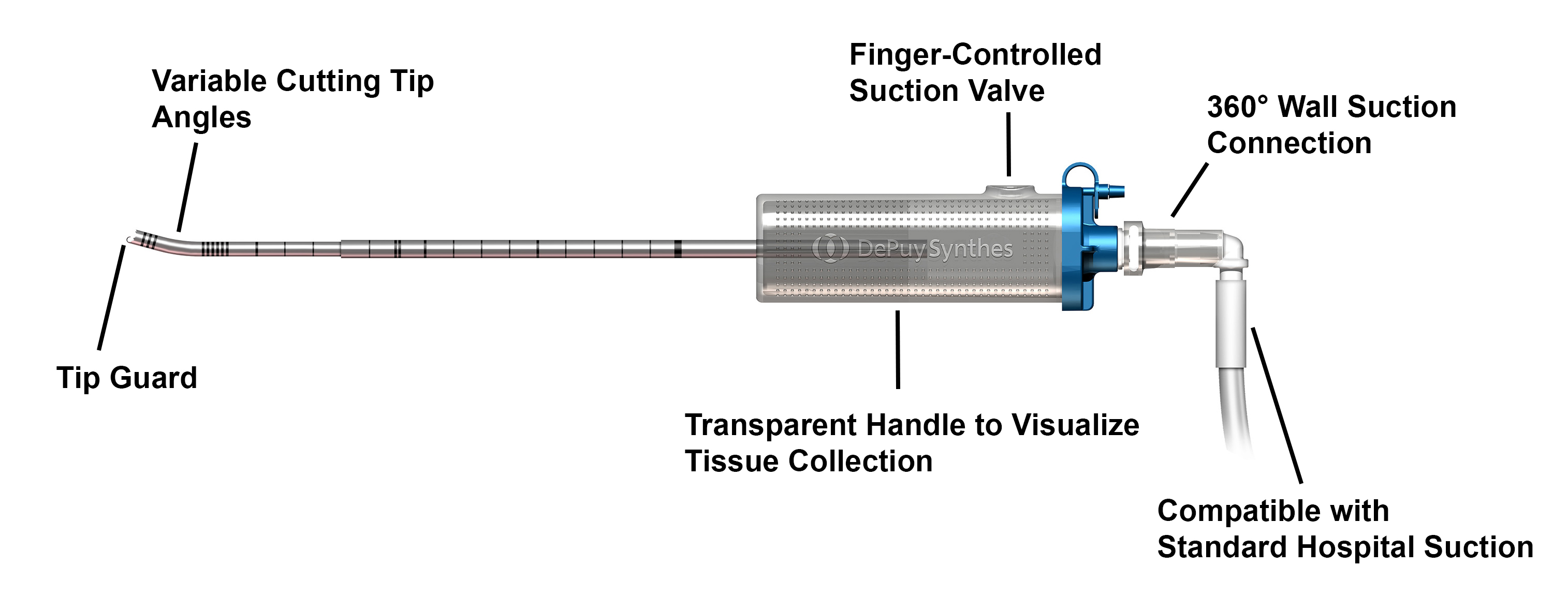
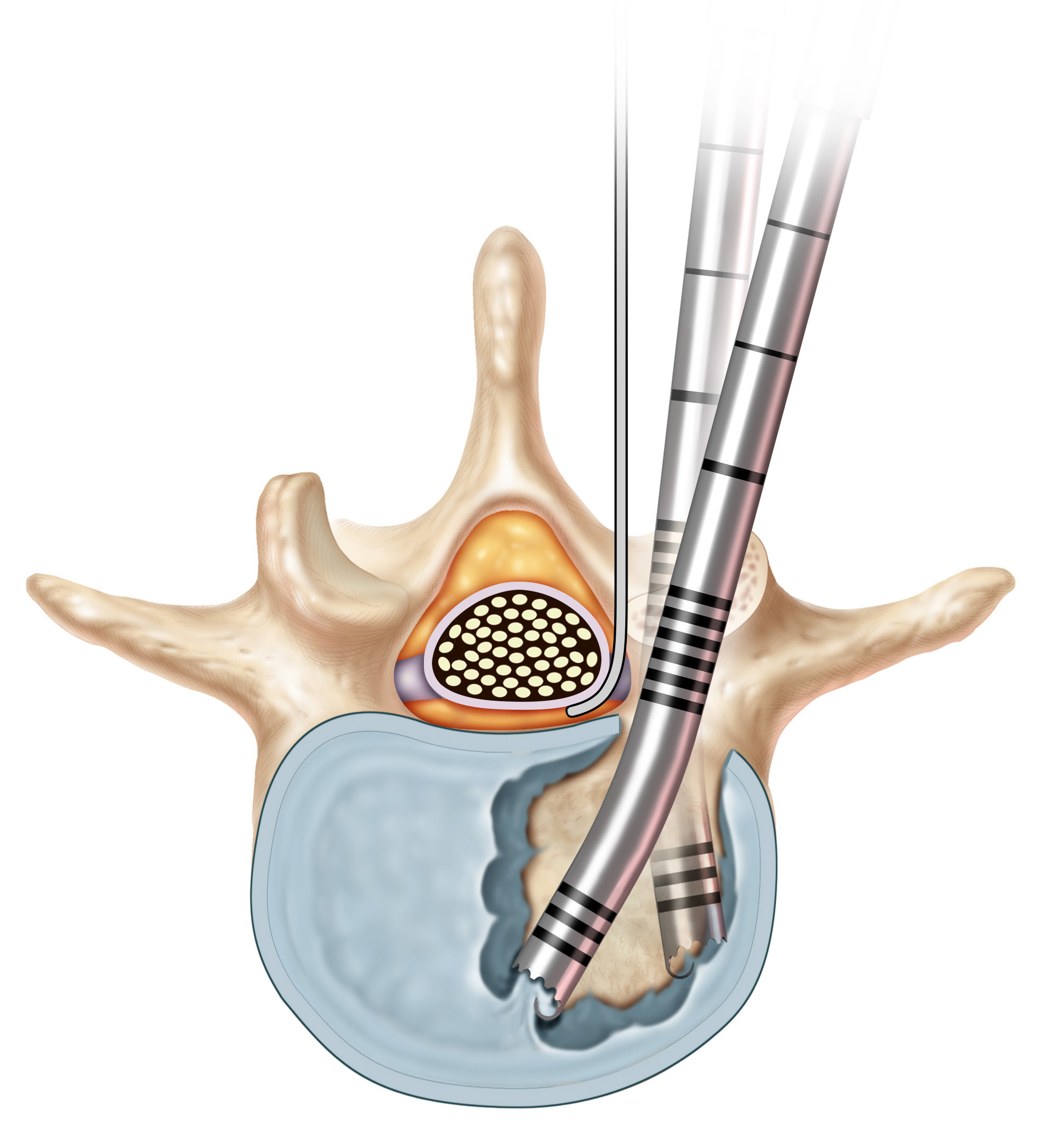
The CONCORDE Clear MIS Discectomy Device is a single-use instrument that removes the degenerated disc and prepares the endplates using readily-available standard hospital suction (Figs 2 and 3). The instrument is available in angles of 15°, 30°, and 40° and two lengths (220 mm, 270 mm) to accommodate various approaches. The diameter of the tube is 5 mm. The cutting edges shear the disc material from the endplates while the suction draws the disc material into the transparent handle, which allows surgeons to immediately see the collection of disc material. This discectomy technique requires fewer tool passes and instrument exchanges when compared to traditional techniques. The time saved from reduced instrument steps/passes may lead to reduced OR costs.
The CONCORDE Clear MIS Discectomy Device is a innovative solutions to meet the increasing demands for MIS in spine surgery.
References
1) Vialle EN, Vialle LRG, Gusmo MS. Transforaminal lumbar discectomy: quantitative study in cadavers. Coluna/Columna. 2009; 8(2):134-138.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.