PROTI 360°
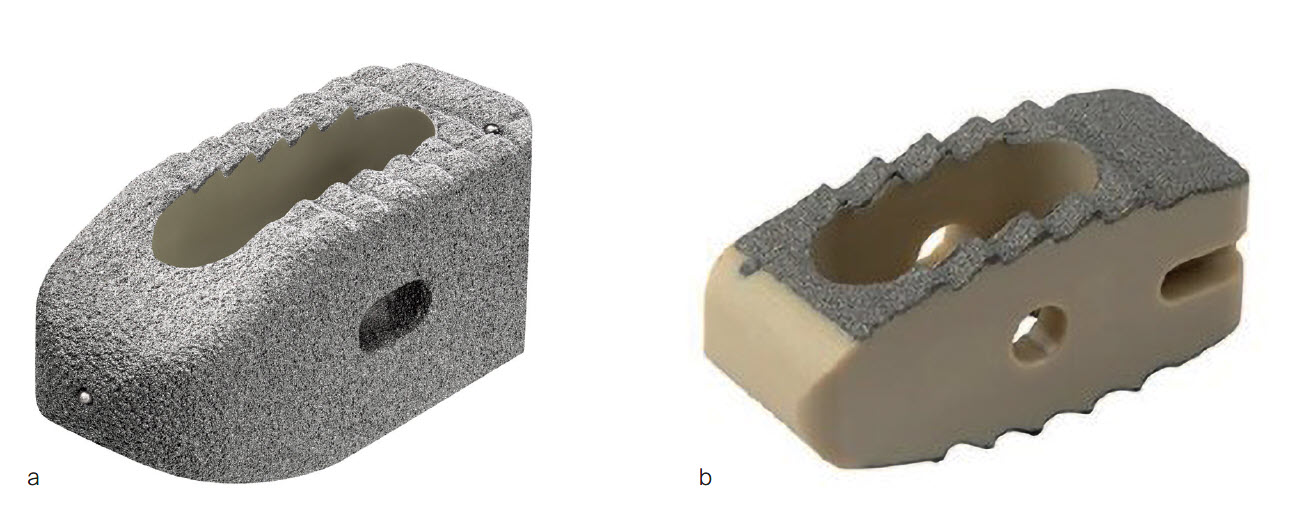
PROTI 360° is a family of integrated titanium interbody cages (Fig 1) that promote spinal fusion, intended for use in patients with degenerative disc disease (DDD). The cage family includes the ACIS PROTI 360° System for cervical fusion, and the T-PAL PROTI 360° System and CONCORDE PROTI 360° Systems for lumbar interbody fusion.
Every year in the US, approximately 400,000 patients with DDD undergo spinal fusion surgery to help reduce pain and nerve root inflammation [1]. During this procedure, the intervertebral disc is removed and an interbody spacer is implanted to potentially restore natural height and lordosis between two vertebrae.
Advantages of PROTI 360 Integrated Titanium implants
The PROTI 360° interbody device has unique design features that provide immediate mechanical stability to the spine and promote rapid and long-lasting biological fixation. The titanium-integrated polyetheretherketone (PEEK) implants combine the benefits of the constituent materials, PEEK and titanium. The integrated titanium on all external surfaces creates a wear-resistant, bioactive surface that promotes the attachment and growth of osteoblasts [2-3], thereby maximizing the potential for bone growth in the intradiscal space. PEEK is a biocompatible material with mechanical properties that resemble human bone. The implants are radiolucent, meaning that surgeons can easily undertake imaging to assess the bone healing process and fusion rate post-operatively [4].
Mechanical stability
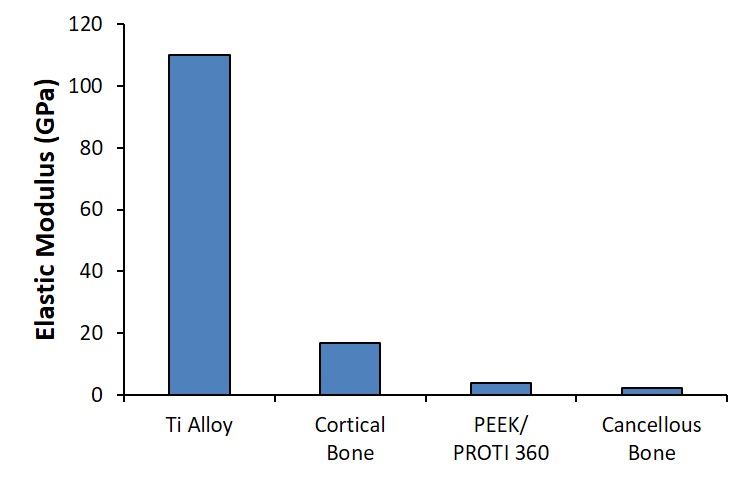
The PEEK core of the interbody device has an anatomically relevant Module of Elasticity, providing mechanical stability and effectively dispersing dynamic loading in the spine to minimize stress shielding effects (Fig 2).
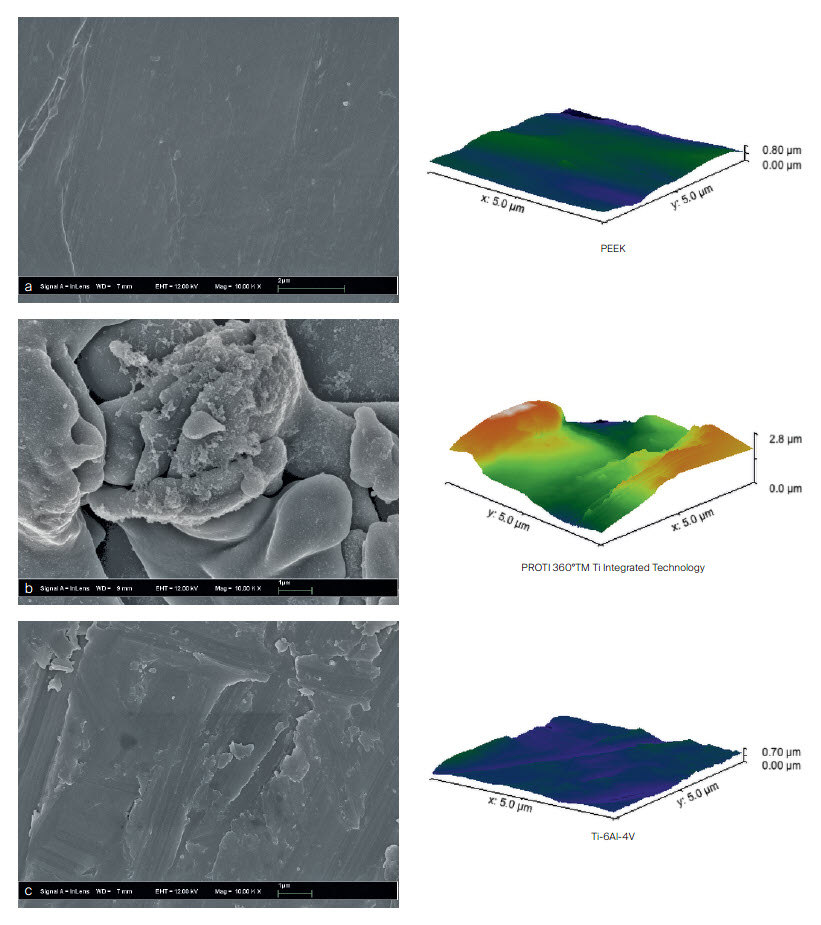
The PROTI 360° cage surface incorporates roughness at the macro-scale intended to improve friction fit within the disc space (Fig 3) [5]. Additionally, roughness at the micro- and nanoscales is intended to enhance cell-substrate interactions to support bone growth (Figs 4,5).
Reduced risk of delamination
The PROTI 360° family of Implants has design features that prevent delamination, a process in which the titanium layer can separate from the PEEK implant at the interface between the two materials. The titanium integrated technology is designed to enhance the Ti-PEEK bonding strength and to reduce the risk of delamination upon impaction (Fig 5). The rounded design of the interbody device eliminates external exposed PEEK corners at leading edges as the device is inserted (Fig 6). The thickness of the porous Ti layer and Ti integrated layer is on average 0.2mm [7]. The manufacturing process for integrated titanium delivers Ti penetration of 223 M along all surfaces [7].
Enhanced bone growth
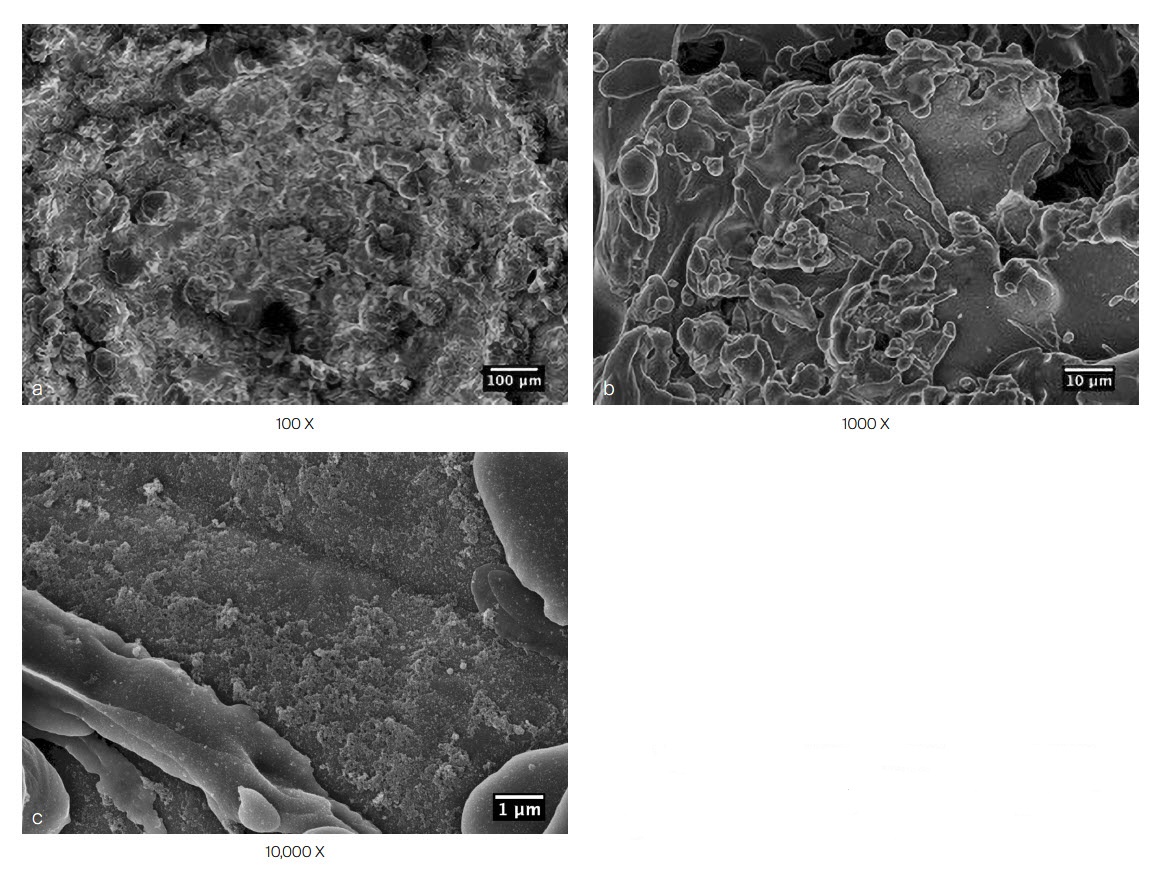
The PROTI 360° cage has an increased surface area with an all-round (360) external integrated titanium surface compared to traditional "endplate coating only" cages (Fig 6), which may enhance bony on-growth during the process of spinal fusion. The integrated titanium surface shows significantly higher osteoblast activity at days 14 and 21 compared to both PEEK and Ti [5]. The increased surface roughness increases the in vitro osteoblast population by ~50% within 7 days compared to standard PEEK surfaces [5]. Calcium deposition, which is an indicator of early bone formation, was significantly higher with PROTI 360° Technology than with both PEEK and titanium alone at days 1 and 7 of device testing [5].
Radiolucency of PEEK
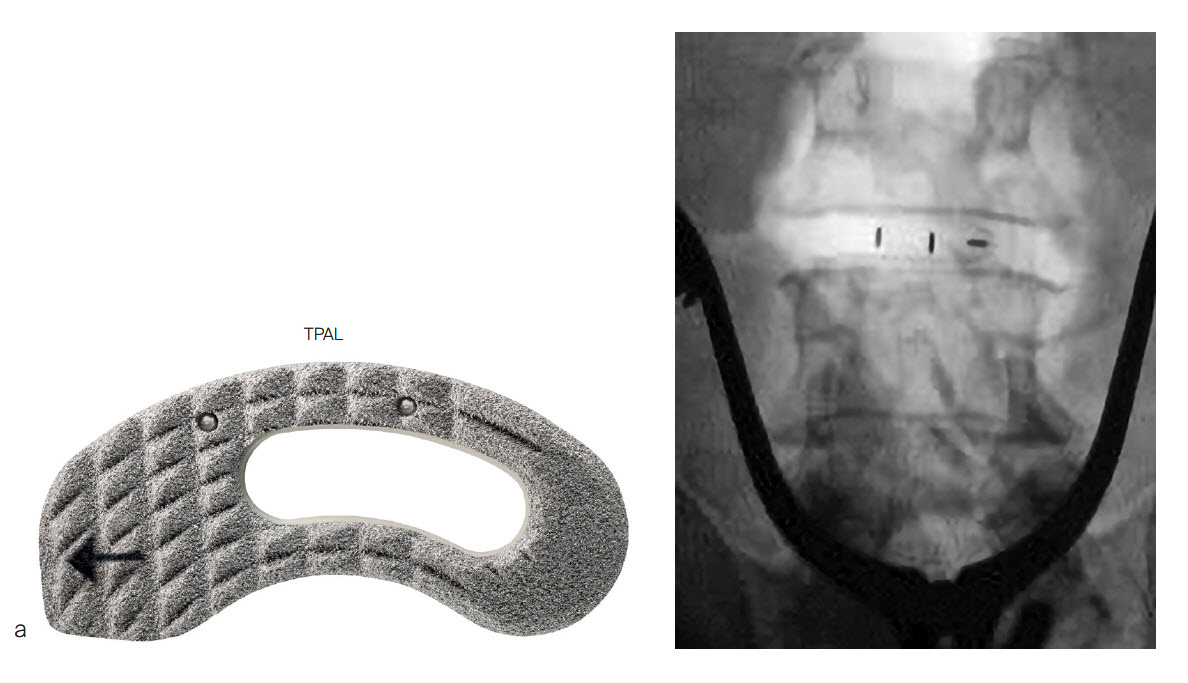
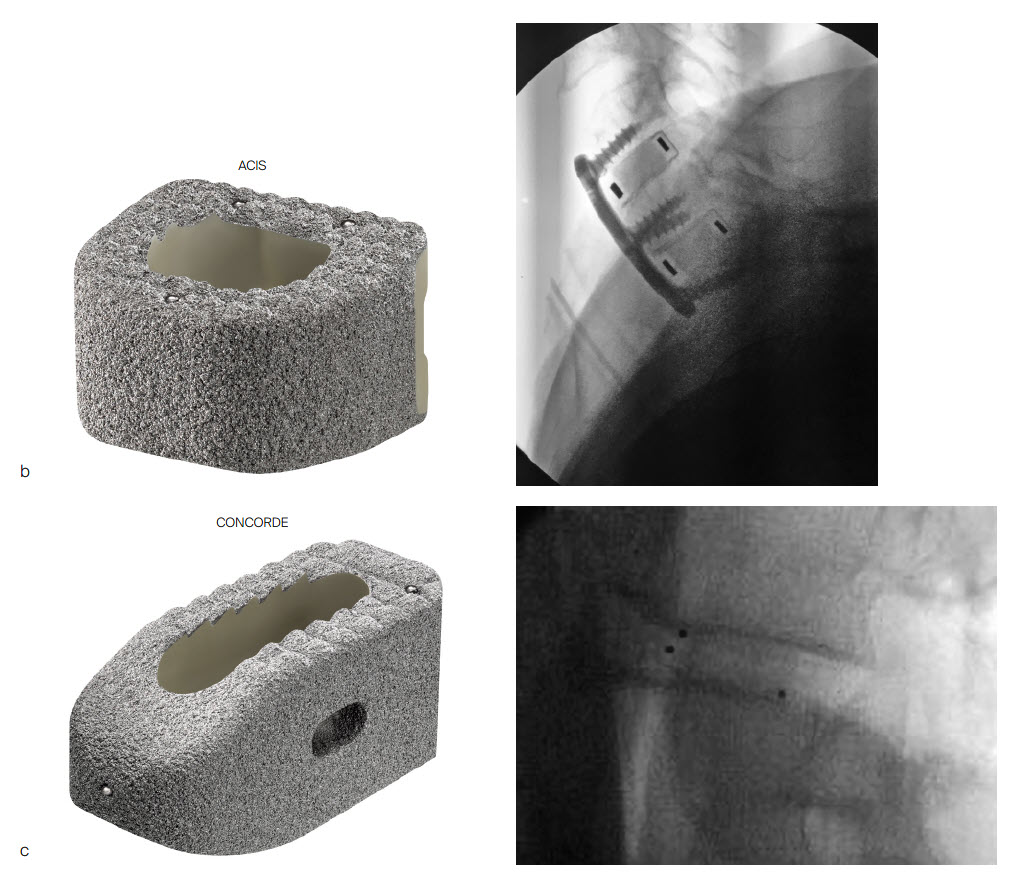
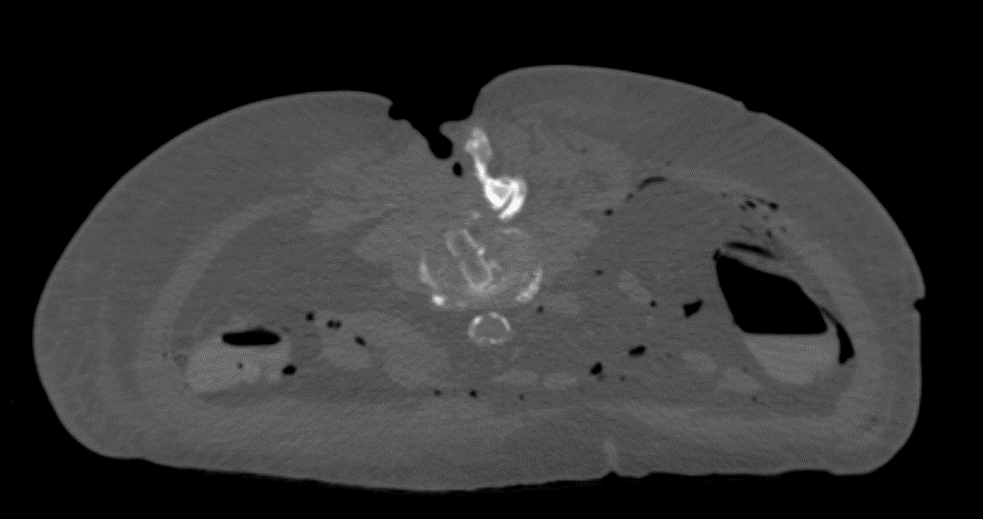
The PEEK core of the PROTI 360° cage has favorable imaging characteristics which support the postoperative assessment of fusion, while the titanium outer layer allows measurement of cage positioning. Clinically, depending on the image quality, plane of view, and patient anatomy, the PROTI 360° cage may show a ghost image of the entire cage on fluoroscopic images (Fig 7). CT images show minimal scatter around the implant (Fig 8).
References
- Rajaee SS Bae HW, Kanim LE, et al. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine (Phila Pa 1976). 2012 Jan 1;37(1):6776.
- Lincks J, Boyanbuthscsa D, Boyan CR, et al. Response of MG63 osteoblast-like cells to titanium and titanium alloy is dependent on surface roughness and composition. Biomaterials.1998 Dec;19(23):22192232
- Kurtz SM, Devine JN. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials. 2007;20(4):278281.
- Nemoto, O., Asazuma T, Yato Y, et al. Comparison of fusion rates following transforaminal lumbar interbody fusion using polyetheretherketone cages or titanium cages with transpedicular instrumentation. Eur Spine J. 2014 Oct;23(10):21502155.
- TR-201801-B (ProTi Test Report).
- Rho JY, Ashman, RB, Turner CH. Youngs modulus of trabecular and cortical bone material: ultrasonic and microtensile measurements. J Biomech. 1993:26(2):111119.
- TR-201803-A (Integrated Titanium Bonding strength Tyber testing report).
Instrument sets and implants
The PROTI 360 cages are available as sterile implants and are compatible with ACIS System (Fig 9a), T-PAL Interbody System (Fig 9c) and CONCORDE Bullet (Fig 9c) instrument sets. The ACIS implants are available in 5-10mm heights, in Lordotic Small, Standard, and Large designs and in Parallel Standard or Convex Standard. The CONCORDE implants are available in Parallel and Lordotic designs (7-15 mm heights, 23 and 27 mm lengths). The T-PAL implants are available in the High Curve design (7-15 mm heights, 10 x 28, 12 x 32 footprints).
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.