New horizons for the DRIM-Nail
The AO Innovation Translation Center (AO ITC) is delighted to provide an update regarding the evolution of the Distal Radius Intramedullary Nail (DRIM-Nail), an implant development project in the early stages of clinical application. Following recent investment activities by DISRAD AG in the wider availability of the DRIM-Nail, a clinical case series has been (re-)launched in selected clinics in Switzerland, Germany, and Austria to enable data collection around device performance and patient outcomes.
Approved by the AO in June 2022, the DRIM-Nail is an innovative treatment option for patients with extraarticular distal radius fractures. The design, development and certification of this new intramedullary nailing implant originally grew from a multi-partner collaboration between DISRAD AG, a start-up founded at Balgrist University Hospital by the Balgrist Beteiligungs AG, the University of Zürich, the AO Technical Commission's Hand Expert Group, the AO's Innovation Funding, and 41medical.
Fig 1: The Distal Radius Intramedullary Nail (DRIM-Nail)
Since obtaining its CE mark—a regulatory milestone that remains in effect—the DRIM-Nail has already been used in the treatment of approximately 50 distal radius fractures to date. In partnership with expert surgeons at the aforementioned clinics, further data collection is currently underway with a focus on two key areas: patient outcomes following treatment with the new implant, and the procedural experience for surgeons. Insights from clinical usage are expected to provide the framework for a future clinical study. Additionally, the DRIM-Nail is anticipated to gain FDA approval in the near future, allowing expansion to the US market.
Key insights from the case series will be presented at the AO ITC’s Innovation Hub on December 8, 2025 as part of this year’s AO Davos Courses. This event will build on last year’s Meet the Expert session (Davos, December 2024), which was an interactive stage show featuring a practical demonstration of the surgical technique to insert the DRIM-Nail and an overview of the clinical benefits of the new device. The presenters included renowned surgeons Andreas Schweizer, Martin Langer and Alejandro Lluch, who were instrumental in the development of the new implant. The session was met with highly positive feedback, confirming its strong reception both in Davos and internationally.
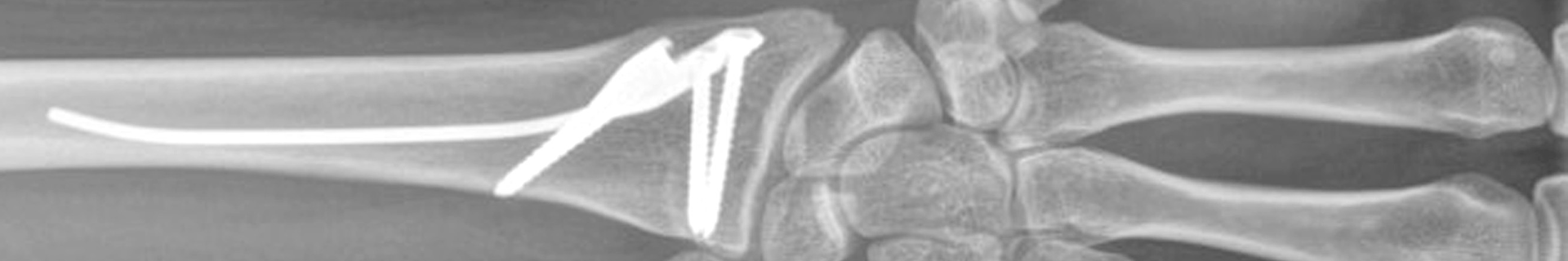
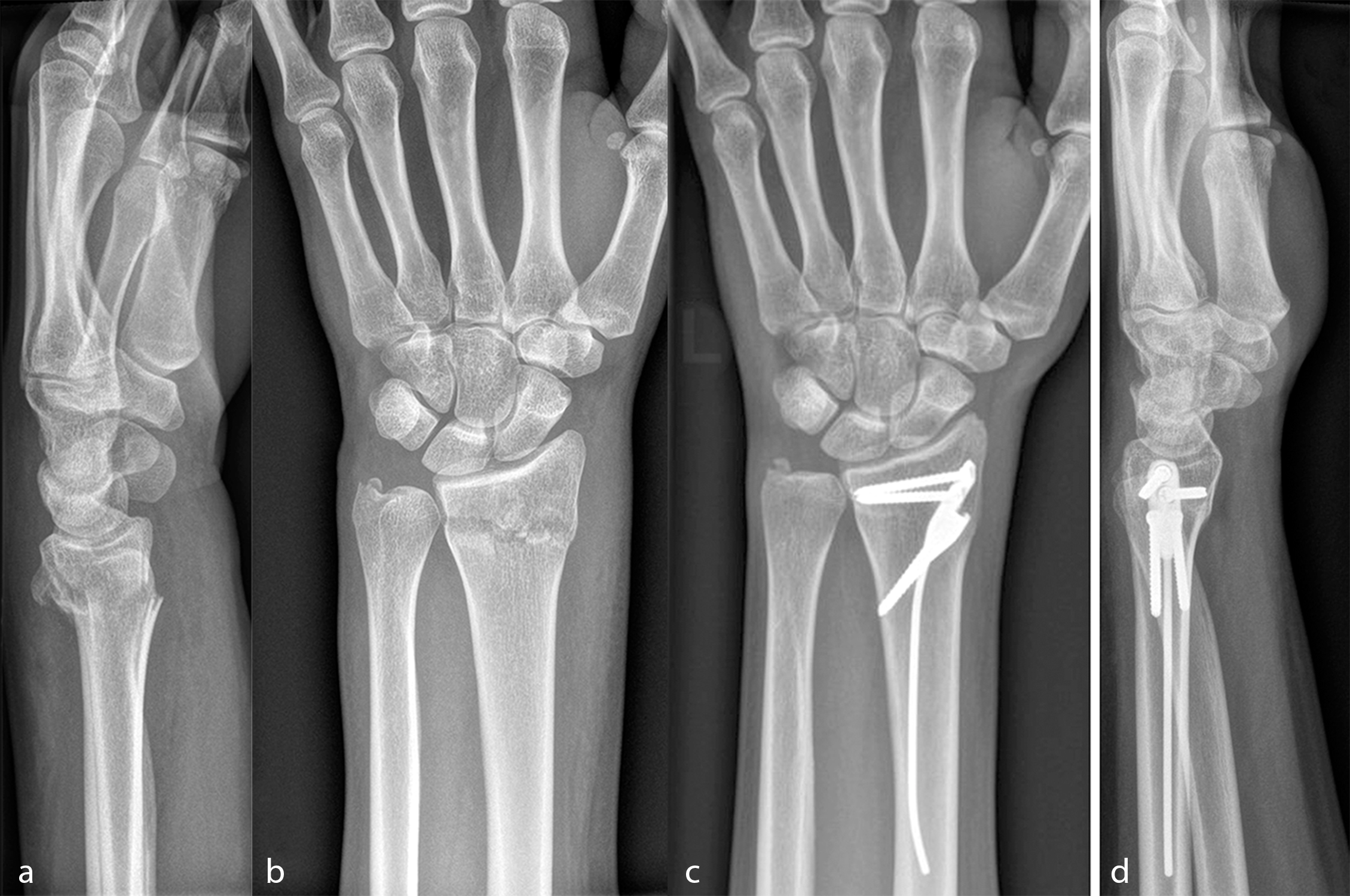
Fig 2: Exemplar case using the DRIM-Nail: (a) Lateral pre-operative radiograph after closed reduction showing a dorsal dislocation of 25° compared to the physiological palmar tilt and a dorsal comminution; (b) AP pre-operative radiograph following closed reduction; (c) AP and (d) lateral radiographs after 3 months with the distal radius fracture healed in anatomical position. (Case kindly provided by Ladislav Nagy, MD, Balgrist Universtity Hospital, Zurich, Switzerland).
Consistent with the AO TC’s ethos of harnessing innovation to improve patient care and outcomes, the new DRIM-Nail provides all the benefits of minimally invasive intramedullary fracture stabilization, including minimal trauma to the bone fragments and the protection of the adjacent soft tissue. This aims to achieve faster recovery, better and faster functional and cosmetic outcomes, and a lower economic burden compared to current treatment options.
Prof. Dr. med. Martin Langer said: “The DRIM-Nail system is the most gentle implant system available - and the surgical time was also shorter than with plate osteosynthesis. This new solution marks a genuine step forward for patient care and outcomes. We look forward to seeing the findings from the clinical case series, and hope that the DRIM-Nail will soon be more widely available for patients and surgeons worldwide.”