Unlocking osteoarthritis: what nearly two million genomes can teach us
BY DR DINO SAMARTZIS AND DR JASON PY CHEUNG

A global genetic analysis involving nearly two million individuals has uncovered hundreds of new genetic links to osteoarthritis of various joints throughout the body. The research offers fresh insights into the disease and new hope for future treatments. Here, key study researchers Dino Samartzis and Jason PY Cheung explain the study’s findings and how they may reshape the way we understand, prevent, and eventually treat osteoarthritis, a debilitating condition that is one of the world’s fastest-growing causes of disability.
-
Read the quick summary:
- Researchers present a 1.9-million-person genomic study mapping genes and pathways that explain osteoarthritis across multiple joints.
- The study found 962 signals and 700 effector genes, revealing drug-repurposing prospects, rare high-risk variants, and shared biology across joints.
- Surgeons can use emerging genetic markers to refine diagnosis, stratify risk, guide surgical timing and selection, and align care with targeted therapies.
- Next steps include diversifying cohorts, validating pathways, testing repurposed drugs, and translating findings into precision, tissue-focused OA treatments.
Disclaimer: The article represents the opinion of individual authors exclusively and not necessarily the opinion of AO or its clinical specialties.
Often regarded as an inevitable part of ageing, osteoarthritis (OA) is not a disease that we can afford to overlook any longer. Surpassed only by dementia and diabetes, OA is the third most rapidly growing health condition associated with disability. According to projections, the disease will affect one billion people globally by the year 2050. In spite of this fast-approaching health crisis, no effective disease-modifying treatments currently exist to target the underlying causes of osteoarthritis and halt its progression.
In order to bridge this gap, it is essential that we better understand the biological processes that lead to this complex disease. Our study, Translational Genomics of Osteoarthritis in 1,962,069 Individuals, which was published recently in the journal Nature, is an attempt to map the genetic architecture of OA in unprecedented detail: in the largest genome-wide association study (GWAS) of osteoarthritis to date and possibly the largest musculoskeletal study ever, we analyzed the genetic data of more than 1.9 million individuals from different populations worldwide.
The study received the esteemed 2025 Research Highlight Award by Arthritis UK and has gathered substantial attention from the community, having been downloaded close to 60,000 times in the first eight months after publication.
Key findings
Our study revealed 962 independent genetic signals associated with osteoarthritis, over half of which—a total of 513—have not previously been reported. We integrated these findings with high-resolution molecular data from joint tissues, including transcriptomic, proteomic, and epigenomic profiles. As a result, we were able to connect these signals to 700 likely effector genes. These genes are not random: many of them converge on key biological pathways. Our cross-disciplinary approach allowed us to go beyond statistical associations and get closer to understanding the biological mechanisms at play in osteoarthritis.
Among our most promising discoveries is the fact that approximately ten percent of the implicated genes produce proteins that are already being targeted by approved drugs today. This suggests that there may be potentials for drug repurposing, which would significantly accelerate the development of disease-modifying therapies.
We also identified rare genetic variants with larger effect sizes than common ones, suggesting that certain individuals may carry high-risk mutations that could eventually inform precision medicine approaches.
Re-evaluating disease pathways
The 700 effector genes implicated in OA biology that we identified—ten times more than previously known—form an unprecedented map of the genetic architecture of osteoarthritis for clinicians and researchers. They are not isolated, but converge across eight key biological processes, suggesting that osteoarthritis arises not from a single disrupted mechanism, but from complex interactions among multiple pathways.
These include TGFβ, WNT, and BMP signaling—pathways that have long been implicated in skeletal development as well as cartilage and bone formation—but also processes that have not previously been genomically linked to osteoarthritis such as the circadian rhythm and glial-cell processes. Retinoic acid signaling emerged as a top driver, with ALDH1A2 (involved in skeletal patterning) and CYP26B1 (regulating retinoic acid degradation) as critical effector genes.
Similarly, circadian rhythm genes like CLOCK and ARNTL revealed unexpected ties to tissue repair and pain perception, while glial-cell pathways highlighted potential targets for modulating OA-related neuroinflammation. The extracellular matrix (ECM) pathway underscored the importance of collagen balance, with variants in COL2A1 and ACAN disrupting cartilage integrity.
Potential boosts to drug development and research
Crucially, as many as one out of every ten of effector genes identified in our research encodes proteins that are already being targeted by existing medications approved for other conditions, for instance TGFβ inhibitors. This means that there may be numerous opportunities for drug repurposing, which could considerably shorten the timeline for therapeutic development and potentially deliver the first disease-modifying osteoarthritis treatments to patients much sooner than if we were starting from scratch.
Our findings also reshape OA as a disorder rooted in developmental biology, cellular metabolism, and neural-immune crosstalk—a paradigm shift that could further accelerate the path to precision therapies.
Thirdly, our study reinforces the critical importance of studying osteoarthritis in the right tissues. Chondrocytes (cartilage cells) and primary joint tissues were especially informative in pinpointing the molecular mechanisms at work. For clinicians and researchers designing future translational studies or biomarkers, this offers a strong signal about where to focus attention.
Clinical takeaways for orthopedic surgeons
Our findings have several key implications for orthopedic surgeons. First, they underscore the biological complexity of osteoarthritis—highlighting why structural changes seen on imaging do not always correlate with symptoms, and why some patients progress rapidly while others remain stable for years. A better understanding of each patient’s genetic risk profile may one day help tailor not only surgical timing, but also perioperative management and adjunct therapies.
Furthermore, we found evidence that rare loss-of-function (LOF) variants carry significantly higher effect sizes than common variants. As sequencing technologies become more accessible, these rare variants could help stratify patients—and perhaps even inform decision-making processes on who might benefit from surgery earlier, and who might benefit more from emerging medical therapies.
Thirdly, our study could add clarity on a genetic level in cases of challenging diagnoses such as spinal osteoarthritis, where overlapping symptoms from disc disease or nerve compression regularly make clinical judgement more difficult. We found that genetic signals for spinal OA overlap with those in other joints, suggesting a shared disease biology. More precise genetic and molecular markers may eventually become key features when it comes to differentiating genuine spinal OA from other spinal pathologies, improving diagnosis and patient selection for both surgical and non-surgical interventions.
Benefits for patients and their families
We believe that our research offers hope for those hundreds of millions of patients who live with osteoarthritis as well as for the healthcare providers who support them. In the past decades, OA treatment has almost exclusively consisted of symptom relief: pain management, physical therapy, and ultimately joint replacement. Our study may play a crucial part in addressing the disease at its root.
Better identifying not just risk genes, but biological pathways and potential drug targets, could become key to the development of novel OA therapies that could slow, halt, or even prevent disease progression. Many of the implicated proteins are already being targeted by approved medications, which means that there is a real chance to quickly progress to clinical trials for at least some applications. The more we understand the genetic profiles that drive OA, the closer get to being able to offer personalized treatment plans that are more than just a temporary fix.
However, more work remains. We acknowledge the current limitations in genetic diversity across our study populations, and we are committed to expanding our datasets to reflect the true global burden of OA. As we continue this work, our aim is clear: to translate genetic knowledge into meaningful clinical advances that improve quality of life for patients across every age group, geography, and stage of disease.
Collectively, our study also emphasizes the importance of team science. To make any headway or innovation in today’s age, working together rather than in silos is paramount. Our study underlines the importance of how bringing together a multidisciplinary team from across the globe can make lasting impact by facilitating giants leaps forward rather than baby steps in understanding and treating OA.
***
Professors Samartzis and Cheung are authors of the publication Hatzikotoulas, K., Southam, L., Stefansdottir, L. et al. Translational genomics of osteoarthritis in 1,962,069 individuals. Nature 641, 1217–1224 (2025). Full list of authors, contributors, and participating consortia is available online in Nature.
About the authors:
Dr Dino Samartzis is a Professor in the Department of Orthopedic Surgery at Rush University Medical Center in Chicago, Illinois. He also serves as the Director of the International Spine Research and Innovation Initiative (ISRII) at Rush.
Prof Samartzis earned his undergraduate degree from Northwestern University. He pursued graduate studies in biological sciences, evidence-based health care, clinical epidemiology, medical sciences, and international studies at institutions including Harvard University, the University of Oxford, the University of Cambridge, Erasmus University, Charles University, and the London School of Economics and Political Science. He completed a postdoctoral fellowship in the Department of Biochemistry at The University of Hong Kong.
He was the Walter Beebe Fellow of the National Academy of Sciences, the National Institutes of Health, and the Radiational Effects Research Foundation (aka, the Atomic Bomb Casualty Commission) in Hiroshima, Japan. He is also a United States Public Voices Fellow and the co-Editor-in-Chief of the European Spine Journal. He is the past chairperson for AO Spine's International Research Commission and past member of AO Spine’s International Board.
Professor Jason Pui Yin Cheung is the Department Chairperson and Clinical Professor of the Department of Orthopaedics and Traumatology at the University of Hong Kong.
After obtaining his MBBS degree from The University of Hong Kong in 2007, Prof Cheung received his training in Orthopaedics at Queen Mary Hospital. He completed his membership examination in 2009 and obtained his Master of Medical Sciences degree from The University of Hong Kong in 2012. He completed his specialist training in 2014. He joined the Department of Orthopaedics and Traumatology as a Clinical Assistant Professor in November 2012 and promoted to Clinical Associate Professor with early tenure in 2018. He obtained his Master of Surgery in 2017, Postgraduate Diploma in Molecular and Diagnostic Pathology in 2018, Doctor of Medicine in 2019, and Master of Education in 2021. He was promoted to Clinical Professor in 2023.
He is the current AO Spine Asia Pacific Chairperson.
You might also be interested in:
Gut health and LDS degeneration
A study has found that gut health may be a factor when it comes to spinal degeneration.
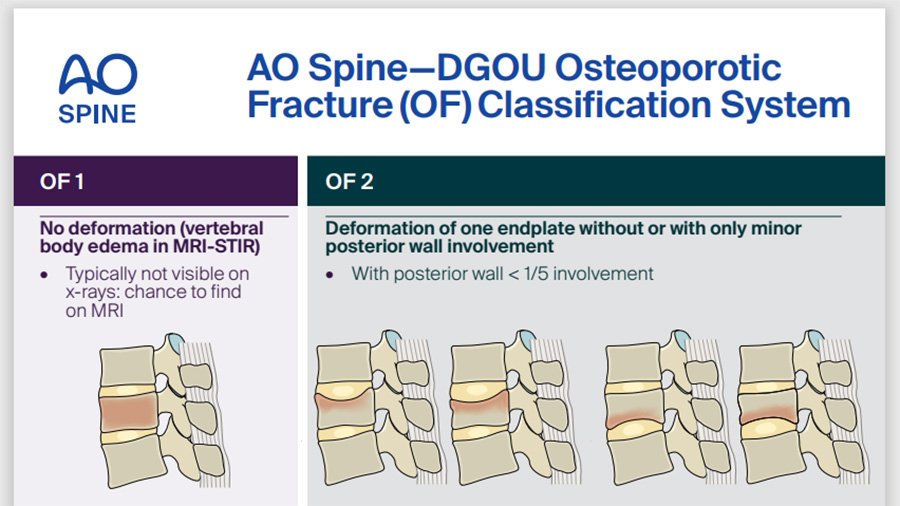
Osteoporotic Fracture Classification System
Download the AO Spine-DGOU toolkit and start using the classification today.
AO Spine research programs
AO Spine sponsors innovative preclinical and clinical studies adhering to the highest standards.