Modic changes: updated insights into back pain, MRI diagnosis, surgical outcomes, and emerging treatments
BY DR PETER M. UDBY
Modic changes (MC) denote a clinically meaningful vertebral endplate–marrow phenotype that co‑evolves with intervertebral disc degeneration. Since Michael Modic’s foundational papers (1988), convergent evidence supports an endplate‑centric, immune‑fibrovascular response that is dynamic (MC1↔MC2) and variably nociceptive. Measurement has matured beyond a three‑type classification to include lesion burden—formalized as the Modic Change Grade (MCG A/B/C)—which offers alignment with patient‑reported disability and possible surgical trajectory.
In nonoperative care, basivertebral nerve ablation (BVNA) is an image‑guided option for appropriately selected vertebrogenic pain (MC1/2) after failed conservative therapy, while routine antibiotics are unsupported outside clinical trials. In surgery, the mere presence of MC seldom dictates indication; however, major MC (MCG-B+) can meaningfully help guide decision‑making—particularly in lumbar fusion and spinal stenosis patients.
The near‑term research frontier is precision phenotyping: integrating cartilage endplate (CEP) imaging, quantitative MRI, and biologically grounded subtypes to better match therapeutic mechanism to patient biology.
-
Read the quick summary:
- Dr Peter Udby reviews Modic changes, their imaging, and clinical relevance in back pain and spine surgery.
- Key takeaway: lesion burden (MCG) is more predictive than type; BVNA is promising, routine antibiotics not supported.
- Surgeons can integrate MCG into surgical planning, refine patient selection, and set realistic expectations.
- Future research explores precision phenotyping, AI MRI analysis, and targeted endplate repair strategies.
Disclaimer: The article represents the opinion of individual authors exclusively and not necessarily the opinion of AO or its clinical specialties.
Background and definitions of Modic changes
MRI classification, grading (MCG), and clinical meaning
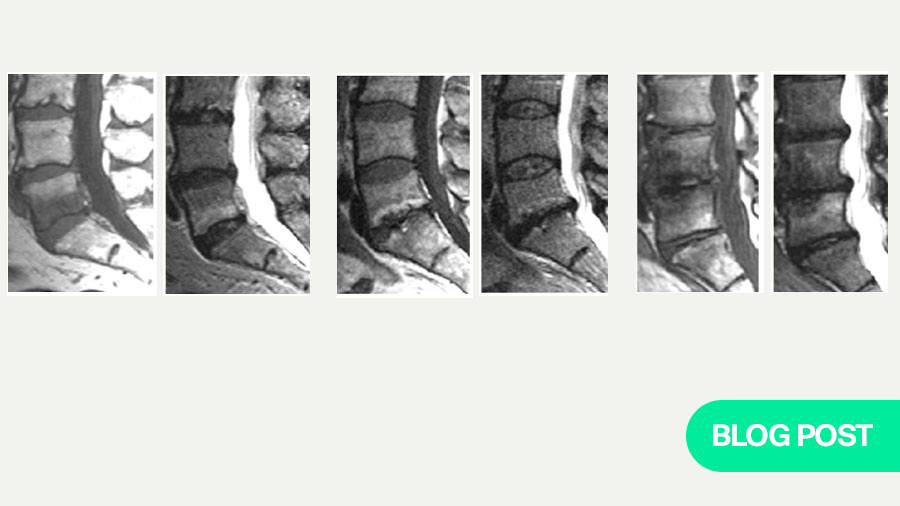
MC are vertebral bone‑marrow signal changes adjacent to the endplate of a degenerating disc. Classic MRI phenotypes are: MC1 (T1 low/T2 high ± STIR hyperintensity; fibrovascular/inflammatory marrow), MC2 (T1 high/T2 iso–high; fatty remodeling), and MC3 (T1/T2 low; sclerosis). Natural history commonly includes MC1→MC2 conversion with occasional progression to sclerosis.
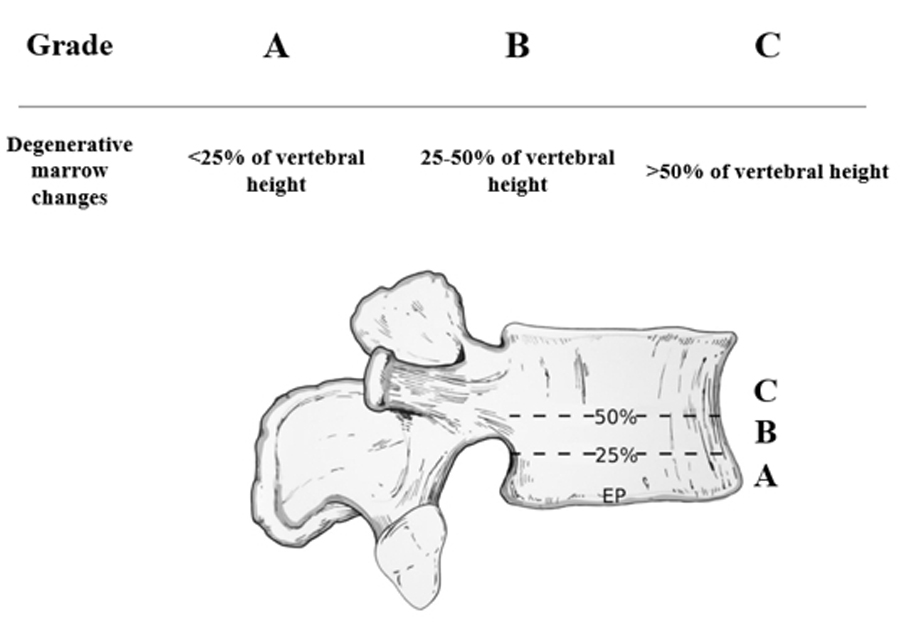
Beyond type, extent matters: the Modic–Udby definition and Modic Change Grade (MCG) categorize vertical involvement—A <25% (minor), B 25–50% (major), C >50% of vertebral body height/volume—enhancing reliability and clinical utility.
Epidemiology and types of Modic changes
Prevalence, phenotypes, and progression in spine degeneration
Prevalence increases with age and overall degenerative burden, with a predilection for lower lumbar levels and the anterior vertebral third. Population‑based cohorts (e.g., ISSLS Prize–winning twin work) link MC to subsequent episodes of severe, disabling low back pain independent of other degenerative features.
Clinically, MC occur more frequently in symptomatic cohorts than in asymptomatic controls. Interpret MC within the degenerative cascade—endplate defects, disc height and signal loss, facet arthropathy, sagittal alignment, and degenerative spinal deformity. Mixed type MCs and interconversion has been described.
Imaging and measurement of Modic changes—from labels to quantitative burden
MRI protocols, lesion burden (MCG), and quantitative methods
MRI protocols should include T1‑ and T2‑weighted sequences with STIR to capture marrow edema. To limit misclassification, standardize sequences and strict structured reporting should be emphasized. Quantitative MRI can refine phenotyping: proton‑density fat fraction (PDFF) differentiates fatty from fibrovascular marrow, and ultrashort‑echo‑time (UTE) MRI improves CEP visualization. Machine‑learning/radiomics are emerging for automated MC detection and extent estimation, enabling reproducible multicentre research and pragmatic registries.
Descriptive reporting template: “L4–5: MC1, MCG‑B, anterior two‑thirds; CEP irregularity; endplate defect present.” This captures type, burden (extent), topography, and the endplate context most relevant to biomechanics and pain.
Pathobiology of Modic changes—endplate–marrow biology and pain mechanisms
Endplate–marrow biology, degeneration models, and bacterial debate
The etiology of MC is likely multifactorial. A consistent observation across cohorts is that MC occur only in the presence of disc degeneration, which strongly implicates degeneration as the primary driver. A unifying mechanobiologic model posits that endplate microdamage impairs cartilage endplate (CEP) transport and permits leakage of disc-derived danger signals into adjacent vertebral marrow. The resultant immune–fibrovascular response manifests as MC1 (heightened turnover/inflammatory signalling), followed by fatty remodelling (MC2) and, in a subset, sclerosis (MC3). Nociception likely arises from chemical and mechanical stimulation of nociceptors within the injured endplate–marrow complex.
The role of bacteria in MC remains uncertain. While some biopsy series report positive sampling (often Cutibacterium acnes) in up to ~70% of samples, most of these data derive from disc tissue—the body’s largest avascular structure—rather than from intraosseous vertebral marrow, which is the actual location of MC. Given the frequent detection of skin commensals, perioperative contamination is a plausible explanation for many “positive” findings. To date, no study has conclusively demonstrated bacteria within the bony vertebra at the MC site, leaving infection as an unproven—and likely minority—pathway relative to degeneration-driven mechanisms.
Clinical Associations with low back pain and disability
Evidence linking Modic changes to symptoms and long-term outcomes
Longitudinal population studies identify MC/endplate damage as independent predictors of severe, disabling LBP episodes. In specialty cohorts, MC1 and higher‑burden lesions correlate more strongly with pain; effect sizes vary with sampling, imaging standards, and analytic approach. Interestingly, in long-term cohort studies (+10 years) of patients with baseline chronic LBP, the presence of MC at baseline did not portend worse outcomes; patients with MC actually demonstrated less disability and fewer sick‑leave days at long‑term follow‑up—reinforcing that label ≠ destiny and that MC biology is dynamic.
Nonoperative management of Modic changes
Conservative care, antibiotics, and basivertebral nerve ablation (BVNA)
Conservative care—education, graded exercise, and analgesia/NSAIDs—remains first‑line for nonspecific LBP and suffices for many patients with incidental or low‑burden MC. Antibiotics: an initial single‑centre RCT suggested benefit of prolonged amoxicillin‑clavulanate in post‑herniation MC1, but the larger multicentre AIM RCT did not demonstrate clinically important benefit; routine antibiotics are not recommended absent clear infection. BVNA: studies including long‑term follow‑up support durable improvements in pain and function for carefully selected patients with vertebrogenic pain and lumbar MC1/2.
Surgical implications of Modic changes
Impact on fusion, discectomy, spinal stenosis, and patient selection
Operative indications are usually driven by neural compression, instability, deformity, and global sagittal balance rather than MC alone. Systematic reviews and meta‑analyses indicate that preoperative MC do not consistently impact outcomes after lumbar discectomy, fusion, or disc arthroplasty. Some series suggest nuanced effects—e.g., early fusion kinetics or implant–endplate interface behaviour may vary with lesion extent/type—but data are heterogeneous. In lumbar spinal stenosis, lesion extent carries prognostic value: major MC are associated with inferior 2‑year VAS‑back/leg and ODI compared with minor MC, despite similar baselines.
Practical algorithms for MRI reading and checklists for clinical decision-making
Structured reporting checklists and treatment pathways
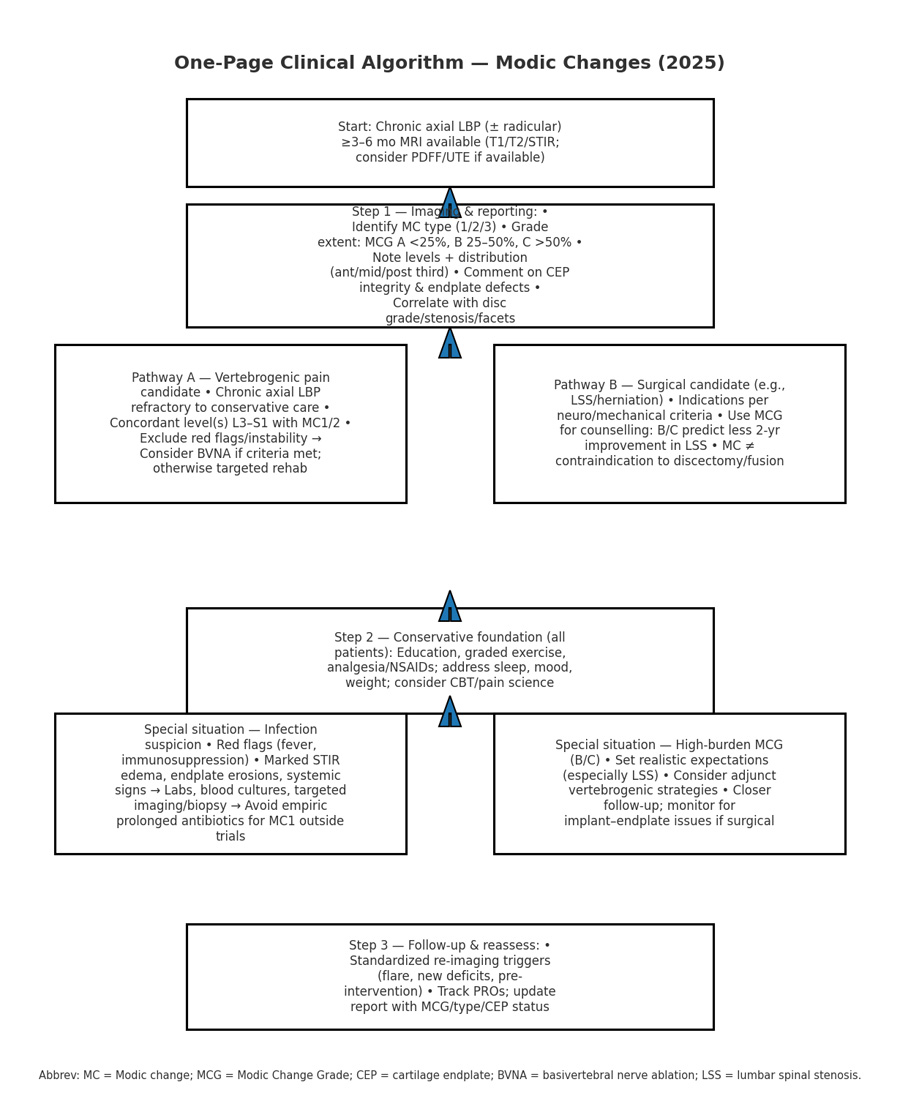
A) MRI reading & reporting checklist
- Modic type (1/2/3) at each level
- Extent: MCG‑A (<25%), MCG‑B (25–50%), MCG‑C (>50%)
- Topography: anterior/middle/posterior third; uni‑/bilateral across midline
- Cartilage endplate (CEP) integrity and bony endplate defects
- Adjacent disc grade; foraminal/central stenosis; facet arthropathy
- Optional quantitative markers: PDFF (%), STIR edema burden
B) Clinic pathway (simplified)
- Chronic axial LBP with MC1/2 at concordant levels → consider vertebrogenic pain phenotype
- Failed conservative care → evaluate BVNA candidacy (inclusion/exclusion per RCT criteria)
- Antibiotics only within trials or with compelling infectious evidence
- For surgical candidates, incorporate MCG into shared decision‑making; set expectations when major MC are present
Quick evidence tables on Modic changes (summary)
MRI types, histopathology, burden grades, and clinical implications
Modic type — MRI signature — Histopathology — Clinical notes
- Type 1 — T1 low / T2 high ± STIR edema — Fibrovascular marrow, high turnover — Often more symptomatic; dynamic; can convert to Type 2
- Type 2 — T1 high / T2 iso–high — Fatty marrow replacement/remodeling — May be less painful on average; prior Type 1 history common
- Type 3 — T1 low / T2 low — Sclerosis — Denotes chronicity; limited near‑term reversibility
MCG Grade — Vertical extent — Typical implications
- A: <25% of vertebral body height/volume — Often incidental/small burden; monitor + conservative care
- B: 25–50% — Higher baseline disability; closer follow‑up; consider targeted options if refractory
- C: >50% — High burden; careful counselling pre‑op; risk of persistent pain; integrate vertebrogenic strategies
- Phenotyping MC1 into bacterial vs. sterile subtypes (culture/omics, immunophenotyping, imaging correlates)
- Quantitative MRI endpoints (PDFF, UTE‑CEP) and automated extent scoring for trials/registries
- Osteoimmunomodulation and endplate‑targeted repair strategies; biomaterials for CEP micro‑repair
- BVNA optimization: imaging selection rules by MCG/topography; health‑economic evaluation across systems
- Prospective surgical decision tools combining MCG with CEP/endplate metrics
- Natural‑history cohorts (dense imaging triggers) and extension to cervical/thoracic MC
The research frontier— future research on Modic changes (2025–2030)
Precision phenotyping, quantitative MRI, immunomodulation, and surgical decision tools
Clinical key takeaways
Modic changes represent a dynamic endplate–marrow process that co-evolves with disc degeneration and can play a significant role in vertebrogenic back pain. For clinicians, the extent of the lesion—captured by the Modic Change Grade (MCG)—is often more predictive of disability and outcomes than type classification alone. In nonoperative care, education, exercise, and analgesia remain the foundation, while basivertebral nerve ablation has emerged as a valuable option for appropriately selected patients with MC1/2 after failing conservative therapy. Routine use of antibiotics is not supported outside of trials.
In surgery, the presence of Modic changes rarely defines the indication on its own, yet high-burden lesions (particularly MCG-B and C) can guide decision-making, shape prognostic conversations, and help set patient expectations—especially in lumbar fusion and spinal stenosis cases. Looking forward, advances in precision phenotyping, quantitative MRI, and endplate-targeted repair strategies promise to refine diagnosis and allow for more tailored interventions. For now, integrating MCG into both reporting and clinical discussions provides a practical framework to bridge imaging with patient care.
About the authors:
Dr Peter Muhareb Udby, MD, DC, PhD is a medical doctor, chiropractor, and PhD with a background in spine surgery and sports medicine. He is based at the Department of Orthopaedic Surgery, Rigshospitalet, University of Copenhagen, where his clinical and research focus spans lumbar spinal stenosis, Modic changes, sagittal balance, and evidence-based surgical decision-making.
With a parallel career in sports medicine, Udby has worked with professional athletes in strongman, martial arts, and power sports. He has served as physician for World’s Ultimate Strongman in Dubai, collaborating with some of the world’s strongest athletes in elite performance and injury management.
Internationally, Dr Udby is a recognized contributor to the global spine community. He has delivered numerous presentations at leading congresses including IMAST, Global Spine Congress, and NASS, where his research has gained wide attention. He was nominated for the White Cloud Award at IMAST and has won Best Paper at NASS, underscoring the academic and clinical relevance of his work.
As an active member of the Copenhagen Spine Study Group (CSSG), Dr Udby leads collaborative, registry-based, and translational projects linking basic science with clinical practice. He is committed to mentorship, education, and global collaboration through AO Spine. His career vision is to unite rigorous science with frontline clinical care—and to integrate lessons from elite sports medicine into spine surgery, advancing recovery, resilience, and patient outcomes worldwide.
References and further reading:
- Dudli S, Fields AJ, Samartzis D, Karppinen J, Lotz JC. Pathobiology of Modic changes. Eur Spine J. 2016;25(11):3723–3734. doi:10.1007/s00586-016-4459-7.
- Velnar T, Gradisnik L. Endplate role in the degenerative disc disease: a brief review. World J Clin Cases. 2023;11(1):17–29. doi:10.12998/wjcc.v11.i1.17.
- Udby PM, Bendix T, Ohrt‑Nissen S, Lassen MR, Sørensen JS, Brorson S, Carreon LY, Andersen MØ. Modic changes are not associated with long‑term pain and disability: a cohort study with 13‑year follow‑up. Spine (Phila Pa 1976). 2019;44(17):1186–1192. doi:10.1097/BRS.0000000000003051.
- Udby PM, Samartzis D, Carreon LY, Andersen MØ, Karppinen J, Modic MT. A definition and clinical grading of Modic changes. J Orthop Res. 2022;40(2):301–307. doi:10.1002/jor.25240.
- Udby PM, Modic MT, Elmose S, et al. The clinical significance of the Modic changes Grading Score. Global Spine J. 2022;Epub ahead of print. doi:10.1177/21925682221123012.
- Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166(1 Pt 1):193–199. doi:10.1148/radiology.166.1.3336678.
- Modic MT, Masaryk TJ, Ross JS, Carter JR. Imaging of degenerative disk disease. Radiology. 1988;168(1):177–186. doi:10.1148/radiology.168.1.3289089.
- Määttä JH, Wadge S, MacGregor A, Karppinen J, Williams FMK. ISSLS Prize Winner: vertebral endplate (Modic) change is an independent risk factor for episodes of severe and disabling low back pain. Spine (Phila Pa 1976). 2015;40(15):1187–1193. doi:10.1097/BRS.0000000000000937.
- Mok FPS, Samartzis D, Karppinen J, Fong DYT, Luk KD, Cheung KMC. Modic changes of the lumbar spine: prevalence, risk factors, and association with disc degeneration and low back pain in a large‑scale population‑based cohort. Spine J. 2016;16(1):32–41. doi:10.1016/j.spinee.2015.09.060.
- Bråten LCH, Rolfsen MP, Espeland A, et al. Efficacy of antibiotic treatment in patients with chronic low back pain and Modic changes (the AIM study): double‑blind, randomised, placebo‑controlled, multicentre trial. BMJ. 2019;367:l5654. doi:10.1136/bmj.l5654.
- Khalil JG, Smuck M, Koreckij T, et al. A prospective, randomized, multicenter study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain. Spine J. 2019;19(10):1620–1632. doi:10.1016/j.spinee.2019.04.009.
- Fischgrund JS, Rhyne A, Macadaeg K, et al. Long‑term outcomes following intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 5‑year treatment arm results from a prospective randomized double‑blind sham‑controlled multi‑center study. Eur Spine J. 2020;29(8):1925–1934. doi:10.1007/s00586-020-06448-x.
- Lambrechts MJ, Issa TZ, Toci GR, et al. Evaluating the impact of Modic changes on operative treatment in the cervical and lumbar spine: a systematic review and meta‑analysis. Int J Spine Surg. 2022;16(4):708–722. doi:10.14444/8285.
- Lambrechts MJ, Issa TZ, Toci GR, et al. Modic changes of the cervical and lumbar spine and their effect on neck and back pain: a systematic review and meta‑analysis. Global Spine J. 2023;13(5):1405–1417. doi:10.1177/21925682221143332.
- Fields AJ, Ballatori A, Liebenberg EC, Lotz JC. Contribution of the endplates to disc degeneration. Curr Mol Biol Rep. 2018;4:151–160. doi:10.1007/s40610-018-0104-6.
- Udby PM, Modic MT, Vestergaard T, Carreon LY. The Modic change grade is associated with patient-reported outcomes in lumbar spinal stenosis surgery. Clin Neurol Neurosurg. 2025 Jul 8;257:109052. doi: 10.1016/j.clineuro.2025.109052. Epub ahead of print. PMID: 40651162.
You might also be interested in:
Courses and events
Explore the upcoming courses, webinars or online events in your region or worldwide.
Global Spine Journal
AO Spine’s official scientific journal is an open access, peer-reviewed journal focusing on the study and treatment of spinal disorders.
AO Surgery Reference
A resource for the management of fractures, based on current clinical principles, practices and available evidence.
AO Spine Classification Systems
Incorporating both fracture morphology and clinical factors relevant for clinical decision making.
AO In-Hospital
A smarter, simpler way for AO Spine faculty to deliver surgical education at their workplace.