AO Fracture Monitor Clinical Trial protocol published in BMJ Open
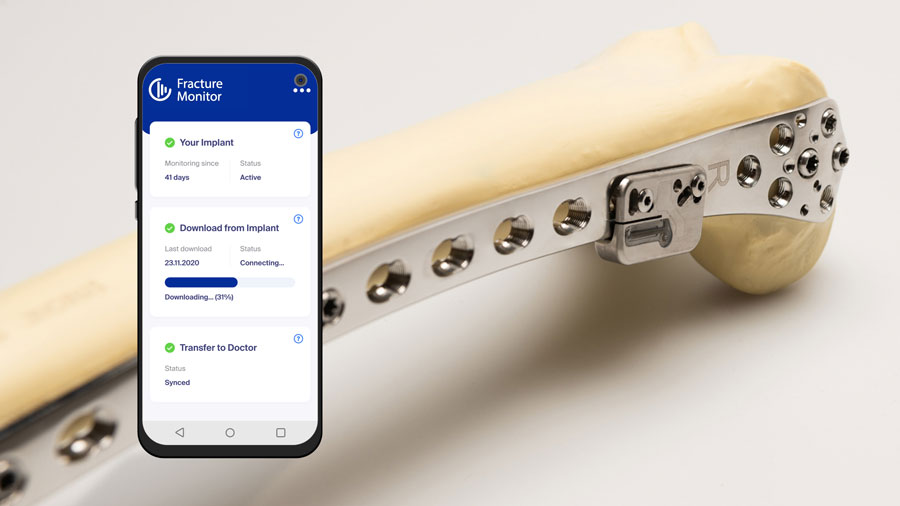
The AO’s pioneering Fracture Monitor project is moving ahead with the recent publication of the clinical study protocol in the respected journal BMJ Open [1]. The clinical study marks a significant step in the validation of the implantable telemetric sensor system designed to continuously monitor fracture healing.
The AO Fracture Monitor, developed through AO Innovation Funding, represents an innovative approach in tailoring rehabilitation to the specific needs of individual patients. By enabling real-time data collection on bone healing, the device aims to optimize post-surgical care and improve patient outcomes.
The ongoing multicenter clinical trial, launched in autumn 2023, is currently underway at four leading German hospitals: BG Klinik Tübingen, Universitätsklinikum des Saarlandes, Universitätsklinikum Ulm, and Universitätsklinikum Münster. To date, 18 patients have been enrolled in this first-in-human, interventional, prospective study, which evaluates the safety of the Fracture Monitor T1 in patients with femoral fracture over a six-month period. 15 of the 18 enrolled patients have already completed their 6-month follow-up. As well as delivering safety and performance data, project stakeholders anticipate that outcome data will advance the surgical understanding of fracture treatment, fracture after-care, and rehabilitation, and how these factors relate to fracture healing.
Manuela Ernst, Senior Project Leader Technology Development at AO Research Institute Davos (ARI), emphasized the importance of this phase:
"This study was rigorously designed and is a critical step toward market availability. The data that we collect will support regulatory approval of the Fracture Monitor under European medical device regulations, meaning that more patients in Europe can benefit from the device.”
The success of the AO Fracture Monitor project reflects a robust interdisciplinary collaboration. AO scientists and engineers spearheaded the device’s development, supported by AO Innovation Funding. Renowned AO surgeons have provided critical clinical expertise and feedback throughout both the development phase and the current initial human implantation phase, while the Clinical Evidence team continues to play a key role in driving the clinical study.
This combination of engineering expertise, surgical skill and clinical insight underscores the AO Foundation’s commitment to advancing trauma and orthopaedic innovation and improving patient care through evidence-based solutions.
Alexander Joeris, Head of the AO’s Medical and Scientific Affairs team, highlighted the unique nature of the study: "This is a landmark study for AO Clinical Evidence – it’s the first pre-market clinical study with an investigational device that our team has designed and implemented." Ramona Ritzmann, Head of the AO's Clinical Operations team, added: "We are proud to support the Fracture Monitor project so that more patients can benefit from this important advancement in fracture care.”
Reference
- Braun BJ, Raschke MJ, Schütze K, et al. Prospective first-in-human clinical investigation to evaluate the safety of the fracture monitor T1 in patients with femur fractures treated with a locking compression plate: a study protocol. BMJ Open 2025;15:e102749.