AO CMF BOOST—Clinical Priority Program
Translational approaches for bone constructs: their impact on facial bone reconstruction
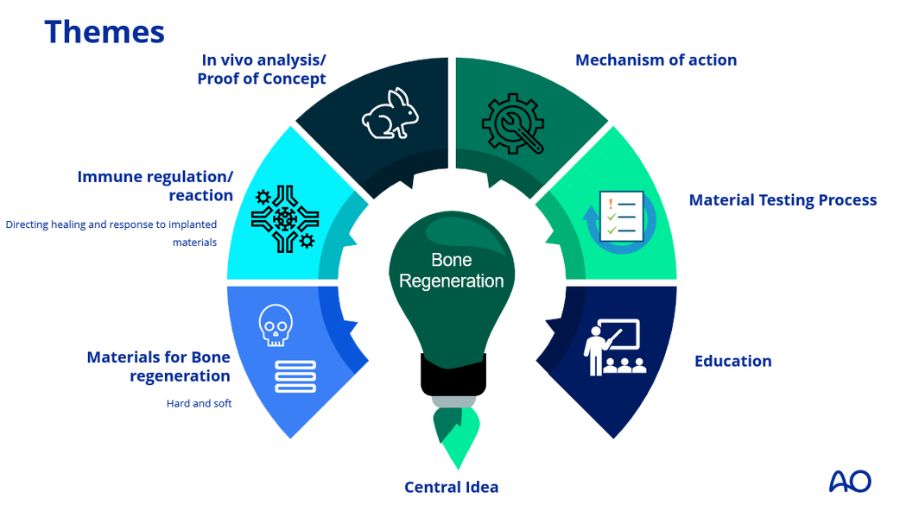
Pillars of the AO CMF BOOST—Clinical Priority Program
-
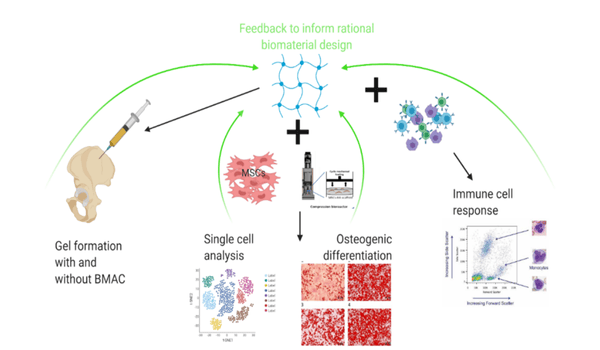
Materials for bone regeneration
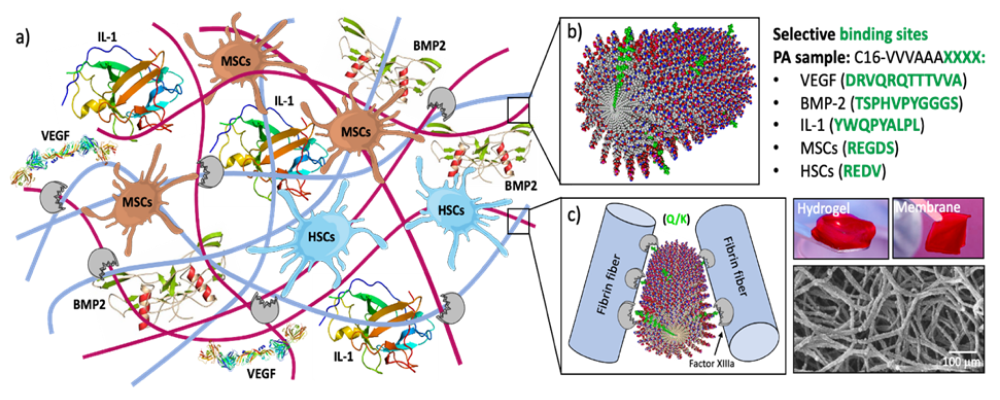
Consortium materials:
- Self assembling Hydrogel (Soft)
- Regulated endogenous factor presentation
- Calcined (heat treated) bone (Hard)
- Particles to improve flat bone healing
- Blocks for larger defects e.g. Mandible
- Flat bone approach
- Gel Sheets patterned with bone particles
- Bulk defects (e.g. Mandible)
- Patient specific bone blocks impregnated with gel
Cellularized intraoperatively with bone marrow aspirate concentrate (BMAC) / fibrin.
Final product: Off the shelf, patient specific, autologous, flexible approach. - Self assembling Hydrogel (Soft)
-
Immune regulation/reactionBackground:
Immune response coordinates and regulates normal healing
In vitro tests lack immunological investigations
Consortium aims:
Monitor
Develop in vitro immunological testing platform to predict in vivo behavior
Establish molecular markers/ screening platform for adverse immunological responses
Control
Regulate immune response using interleukin binding peptides
Eliminate foreign body reactions
-
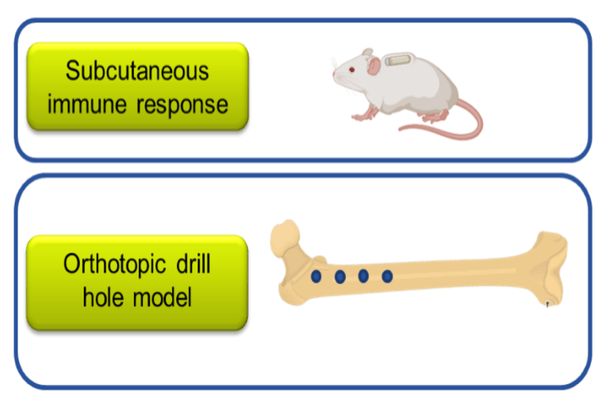
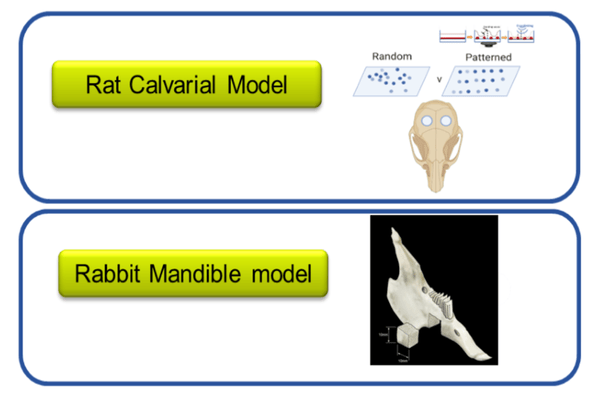
In vivo analysis / Proof of concept
- Screening
- Immunological reactions
- Mechanism of action
- Proof of Concept
- Intramembranous healing
- Endochondral/ bulk healing
-
Mechanism of action
Background:
Understanding of mechanism of action increases potential success of regulatory approval.
Consortium Aims:
Single cell sequencing to establish osteogenesis pathway
Single cell sequencing to interrogate immune response
Modifying effect of mechanical stimulation for mandible regeneration- In vitro bioreactors
Iterative approach to inform material design.
-
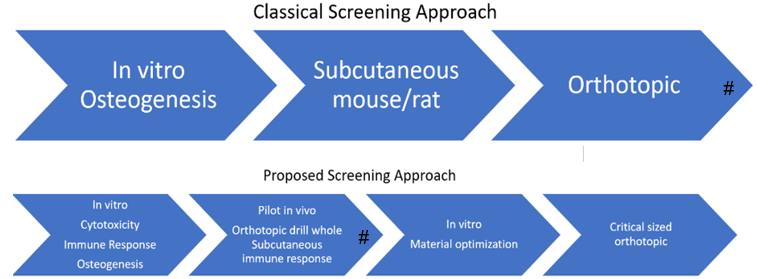
Improved Material testing processBackground:
Many materials show in vitro promise yet fail in vivo
Current classical testing process established decades ago
Consortium aims:
Earlier in vivo tests “fail fast”
Focus optimization on promising compositions
Changing the face of osteogenic material testing -
Education
Consortium aims to enhance performance of Fibrin, BMAC and bone void fillers
Both are currently clinically available
AO Course Lectures:- Fibrin/ BMAC use
- Bone fillers
- Potential for enhancement of clinically available therapies
- Ligorio C., Tavasoli E., Karaman-Jurukovska N., Ittycheri A., Wu Y., Kotowska A.M, Scurr D.J., Gupta S.A., Moogan L.V., Emmetsberger J., Lu F., German G.K., Mata A., Mammone T. (2024). Non-Invasive Monitoring of Palmitoyl Hexapeptide-12 Interaction with Human Skin Layers and Its Cosmetic Skincare Benefits. ACS Applied Bio Materials (Accepted).
- Padilla-Lopategui S*., Ligorio C*., Bu W., Laurenza D., Redondo-Gomez R., Owens R., Iskratsch T., Sun H., Rose F., Mata A. (2024). Biocooperative regenerative materials by harnessing blood-clotting and peptide self-assembly. Advanced Materials, 2407156. https://doi.org/10.1002/adma.202407156. *Equal contribution.
- Ligorio C., Martinez Espuga M., Laurenza D., Hartley A., Rodgers C.B., Kotowska A.M., Scurr D.J., Dalby M.J., Ordóñez-Morán P., Mata A. (2024). Disassembly of Self-Assembling Peptide Hydrogels as a Versatile Method for Cell Extraction and Manipulation. J Materials Chemistry B, 12, 11939-11952, https://doi.org/10.1039/D4TB01575D.
- Murphy J.F., Lavelle M., Asciak L., Burdis R., Levis H., Ligorio C., McGuire J., Polleres M., Smith P., Tullie L., Uribe-Gomez J., Chen B., Dawson J., Gautrot J., Hooper N., Kelly D., Li V., Mata A., Pandit A., Phillips J., Shu W., Stevens M., Williams R., Armstrong J., Huang Y.Y.S. (2024). Biofabrication and Biomanufacturing in Ireland and the UK - Frontier Research for Industry 4.0. Bio-Design and Manufacturing 7(6), 825-856 https://doi.org/10.1007/s42242-024-00316-z.
- Ligorio C., Kotowska A., Scurr D.J., Tavasoli E., Karaman-Jurukovska N., Mata A., Moogan L., German G., Lu F., Mammone T. (2024). 084 Cosmetic peptide penetration and assembly in human skin: A label-free approach, Journal of Investigative Dermatology, 144, 8, Supplement. https://doi.org/10.1016/j.jid.2024.06.100.
- Watts J.A., Ligorio C., Mata A., Fay M.W. Direct detection camera as an alternative to negative staining for peptide structure determination. Proceedings of the Microscience Microscopy Congress 2023, incorporating EMAG 2023. www.doi.org/10.22443/rms.mmc2023.238.
- Ligorio C., Mata A. (2023). Synthetic extracellular matrices with function-encoding peptides, Nature Reviews Bioengineering, 1:518–536. https://doi.org/10.1038/s44222-023-00055-3.
- European Society for Biomaterials (ESB), Turin, Italy, September 7-11, “Influence of Surface Coatings and Topography on Neutrophil Activation and Its Downstream Effects”. Oral presentation.
- Swiss Society for Biomaterials and Regenerative Medicine, Lausanne, 21+22 August 2025, “Shaping Polymers Into Cell-Instructive Constructs for Musculoskeletal Applications”. Invited talk.
- Tissue and Cell Engineering Society (TCES), Bristol, UK, June 18-20, 2025. Plenary lecture.
- Peptide Materials, Gordon Research Conference (GRC), Pomona, California, January 19-24, 2025.
- NIHR MSK, Surgery, Inflammation and Recovery Conference, 3rd October 2024, Nottingham, UK. “Designing Functional Bone Marrow Niches”
- BioMedEng24 at Queen Mary University of London, 5th – 6th September 2024, London, UK. “New tissue engineering opportunities of fibrin materials via innovative assembling and biofabrication strategies”
- BioMedEng Conference 2024, London, UK, September 5, 6, 2024. Keynote lecture.
- Master in Medical Technologies, Applied Biomaterials and Nanotechnologies: Biomaterials development and analytical technologies, 3rd June 2024, Università del Piemonte Orientale, Novara, Italy. “Peptides as molecular building blocks for regenerative biomaterials”
- World Biomaterials Congress, Daegu, Republic of Korea, May 29, 2024. Keynote lecture.
- World Biomaterials Congress, Daegu, Republic of Korea, May 27, 2024. Invited talk.
- Materials Research Society (MRS) Fall Meeting, Boston, November 30 – December 5, 2023. Keynote lecture.
- 14th Berlin School for Regenerative Therapies (BRST) Symposium, 6th - 8th December 2023, Berlin, Germany. “Designing multifunctional biomaterials through peptide-protein co-assembly and acoustic fabrication”
- The Regenerative Games: Uniting the Worlds of Tissue Regeneration in Berlin, December 7, 2023. Invited talk.
- BDI Annual Research Symposium, Talk for the "Regenerating & Modelling Tissues" Theme, 22nd September 2023, Nottingham, UK. “Harnessing peptide-protein co-assembly to engineer multifunctional biomaterials”
- 3rd Midlands Materials Chemistry Meeting, 27th July 2023, Nottingham, UK. “Harnessing peptide-protein co-assembly to engineer multifunctional biomaterials”.
- Tissue Engineering and Regenerative Medicine International Society (TERMIS), Manchester, UK, March 28-31, 2023. Keynote lecture.
- First Summer School of SUPRALIFE EU: Functional Supramolecular Polymeric Biomaterials, Aveiro, Portugal, March 19-21, 2023. Plenary lecture.
- VI RSEQ Chemical Biology Group Meeting – ChemBioVI, Valencia, March 6-8, 2023. Invited talk.
- NIHR Musculoskeletal, Surgery, Inflammation and Recovery Theme Meeting, 23rd November 2023, Nottingham Biomedical Research Centre, Nottingham, UK. “Harnessing peptides to engineer regenerative biomaterials”
- BDI Annual Research Symposium, 22nd September 2023, Nottingham, UK. “Harnessing peptides to engineer regenerative biomaterials”
Over 350 national and international news outlets:
TV
- BBC News
- Bulgarian TV: https://www.linkedin.com/posts/viktoria-petrova-76623530_възможно-ли-е-от-собствената-ни-кръв-да-бъде-activity-7271088453520445440-jzzi/
- The BBC aired a TV program in the Arab World on April 13, 2025
Radio
- The London Evening Standard: https://podfollow.com/tech-science-daily/episode/4002a187bc9bc8d32a23479f60fb4af5af945d17/view
- Hits Radio (East Midlands)
Press
- The Independent: https://www.independent.co.uk/news/science/patients-scientists-university-of-nottingham-blood-b2647657.html
- BBC News: https://www.bbc.com/news/articles/c4g20y29wrvo
- The Jerusalem Times: https://www.jpost.com/science/science-around-the-world/article-829466
- Ireland: https://www.irishexaminer.com/world/arid-41523479.html
- Italy: https://www.fortuneita.com/2024/11/16/dal-sangue-nuovo-biomateriale-per-riparare-ossa-e-ferite/
Grants acquired resulting from AO CMF work
- EPSRC
- Research and Partnership Hub for Health Technologies in Manufacturing Stem Cells (Mainstream)
- January 2025 – December 2029
- £11M. Our group receives £150,000
- Human Frontier Science Program
- 3D-Bioprinting Meets Machine Learning: A Novel Tool for Decipher the Determinants of Viral Tropism
- January 2025 – December 2027
- £350,000. Our group receives £90,000
- Nottingham Biomedical Research Center (NIHR-BCR)
- Development of an in vitro model for athrofibrosis
- January 2024 – December 2025
- Our group receives £80,000
The Team
Consortium Team
Prof Cezmi A. Akdis, MD, PhD
Swiss Institute of Allergy and Asthma Research (SIAF), Co-PI
Prof. Zhiyu Zhou, MD, PhD
The Seventh Affiliated Hospital, Sun Yat-sen University, Co-PI
You might also be interested in
-
AwardsDetails are coming soon
-
MeetingsDetails are coming soon
Latest news
-
Governance
-
Education
 January 16, 2026
January 16, 2026Get ready for an unparalleled educational experience
AO Regional Courses Latin America will be held in Santiago, Chile in August 2026. Early bird offer is available until February 14.
-
Governance
Sign up for the AO CMF newsletter
Do you want to stay up-to-date with the latest breakthroughs in craniomaxillofacial research, education, and patient care? Sign up now and gain insights to exclusive content from leading experts, receive the latest updates on upcoming events, educational or funding opportunities, and never miss a beat in the world of cmf.
Contact us
Do you have questions or need more information?