Bone regeneration using tissue engineering and CAD-CAM technology
Background
Ablative surgery or major facial trauma in the maxillofacial area leads to bone defects that predispose functional as well as aesthetic complications. The bone defects of the facial skeleton need immediate reconstruction to provide satisfactory function of the jaws as well as an acceptable aesthetic outcome. Reconstruction of the maxillofacial area with composite microvascular flaps is challenging and needs a team with experienced surgeons. The surgery is time consuming not only because of the microvascular procedures themselves, but due to demand for optimize intra-operatively the configuration and symmetry of the facial skeleton.The development of three-dimensional (3D) computerized modelling in medicine has been rapid during the last years. However, the use of above mentioned CAD-CAM (computer aided design and manufacturing) is still limited.The technology gives tools for surgeons to plan and to train virtually for the surgery, to design, and manufacture the implants needed for the surgery.Bone tissue engineering requires several constructive factors. These include among others biocompatible scaffolds and matrices, cells, osteo- and angioinductive growth factors. Modern 3D CAD-CAM technology enables patient-specific scaffold and matrix manufacturing. The scaffold, matrix or implant temporarily replaces the missing part of jaw and allows cells to generate bone accordingly leading to anatomic and symmetrical restoration. Increasing number of studies, both experimental and clinical, are available that show bone regeneration in facial bone defects using CAD-CAM technology, patient-specific matrices and cells.
Project Requirements
The use of CAD-CAM and tissue engineering for facial bone defect repair with regard to the outcome of orthopedic, oral and maxillofacial procedures is not well established. In spite of clear development of CAD-CAM technology in medicine and regenerative medicine in surgery, modern facial reconstructive surgery will not be possible without focused high quality, multispecialty research both in translational and clinical level.
For example:
- What are optimal materials and/or bone substitute materials for additive manufacturing (CAD—CAM) for scaffolds, matrices and implants?
- What are the critical stages in CAD-CAM process in medicine?
- Are added cells in bone regeneration in clinical settings necessary?
- Bone defect repair and direct (digital) manufacturing technologies in clinical settings?
AO CMF Funded Research Projects
The abstracts of the latest funded projects under the bone regeneration topic
-
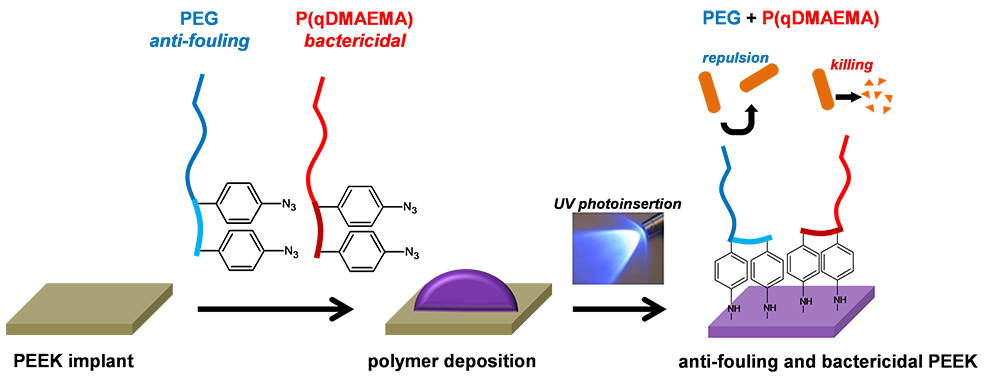
AO CMF-17-02N Project title: PEEK CAM implant with anti-bacterial protectionThe introduction of polyaryletherketone material (PEEK), almost two decades ago had a tremendous impact in orthopedics. Due to its excellent biocompatibility, its inertness, strength and its processability, PEEK-based implants are overtaking many other conventional materials. Importantly, PEEK prostheses can easily be manufactured using modern 3-D printing technology, using data originating from patient's CT scan. As a consequence, fabrication of customized patient implants for craniomaxillo-facial reconstruction made of PEEK has been recently reported, with a relatively good success in terms of aesthetic and biological functionality. Patient specific implants (PSI) made of PEEK are already one of the best choices to reconstruct large and complex defects, when autologous bone graft availability is insufficient.
Unfortunately, PEEK-implant contamination by bacteria is still the main complication and source of printed implant failure, even though antibacterial therapy is administered. The real superiority of such PSI will be realized only if this technology brings advantages for the patient, and not only aesthetically, but also in terms of post-implantation complications. In order to prevent such complications, most researchers have focused their attention in mixing an antibacterial agent with granules of PEEK before implant fabrication. Nevertheless, this option leads to generation of inhomogeneous implants in the bulk with rough topography on the surface which can favor bacteria attachment. Importantly, such techniques cannot be applied on prefabricated PSI, without altering the implant property in terms of mechanical stability and surface topography.
We propose to develop a one-step chemical functionalization which can be applied to any preformed or commercial PEEK PSI that will bring long-term antibacterial protection. By using two different bioactive polymers, we confer to PEEK implants antifouling and bactericide activity. We hypothesize that by combining both approaches in one single coating, we will not only decrease the surface propensity to be colonized by bacteria, but it will actively kill them and clear debris away from the surface of the implant. -
AO CMF-17-17G Project title: MACRON: Mandibular Condyle Regeneration
Maxillofacial bone defects can be difficult to reconstruct. Their shapes are very specific and differ among patients. One particularly challenging reconstruction is that of the temporomandibular joint (TMJ). The top part of the lower jaw, the mandibular condyle, consists of bone tissue covered with fibrocartilage. Gold standard treatment of mandibular condyle defects involves harvesting of a rib from the patient that is then re-shaped to fit the defect. Besides the unpredictable growth potential of this tissue, a second surgical site in the patient is undesirable. Also, numerous attempts have been made reconstructing the TMJ with synthetic materials, such as titanium and biopolymers. However, these had no to limited success. In a search for an alternative regenerative treatment option, several state-of-the-art techniques can be employed.
Biofabrication encompasses multiple techniques that can structurally recreate lost tissue shapes by mirroring scans of the contralateral site. Fused deposition modeling can be used to construct a strong basis that stimulates bone formation. On top, a melt electrospun fibrous mesh can be deposited and be infused with a bioink containing cells or factors that support fibrocartilage formation. Furthermore, recently, the importance of cell origin in tissue reconstruction has been shown. As all skeletal craniofacial structures AO Research Institute Davosse from neural crest derived cells, those are likely to perform best in this context. One of these stem cell populations can be harvested from the dental pulp tissue inside teeth (DPSCs). We have shown these are able to create fibrocartilaginous tissue in our unpublished results. Finally, the biomechanical environment in a joint will dictate local tissue growth and maturation. With novel bioreactor systems, we can improve our understanding of this phenomenon.
With this proposal, we aim to 3D manufacture a biofunctionalized osteochondral mandibular condyle. First, we will design the optimal 3D geometry of the biphasic construct. Second, we will identify the optimal bioink composition for fibrocartilage regeneration with DPSCs. Third, we will improve our understanding of how DPSCs respond in terms of differentiation to mechanical loading in the TMJ.
To achieve these aims, we have assembled a multidisciplinary team of experts consisting of surgeons, engineers and a biologist. Together, we will strive towards 3D biofabrication of clinically translatable MAndibular Condyle RegeneratiON, or MACRON. -
AO CMF-17-19M Project title: 3D printed osteogenic and hierarchical biomineralizing scaffold for bone regenerationThere is great need to develop strategies to improve quality and speed of bone repair in maxillofacial applications. This proposal aims to develop a personalized bioactive 3D printed scaffold with the capacity to significantly enhance bone regeneration and tissue-implant integration. The strategy integrates easy of fabrication, shaping, and handling from 3D printing technology with a novel bioactive material designed to enhance osteoinductivity and osteoconductivity. Specifically, the 3D printed biodegradable polymeric structure coated with the bioactive material will exhibit a personalized and optimized architecture and toughness as well as a peptide-based matrix designed to enhance tissue ingrowth into the scaffold and form highly organized hydroxyapatite growing from the scaffold. The main novelty of this approach consists of creating a personalized bioactive biodegradable 3D printed implant designed to stimulate tissue ingrowth and integration from both the tissue into the scaffold and from the scaffold into the tissue. The project will consist of a) fabricating the anatomically-designed polymeric 3D printed scaffolds, b) coating the scaffolds with a thin bioactive matrix, c) validating bioactivity of the scaffold in vitro by assessing osteoblast adhesion and differentiation using mesenchymal stem cells as well as hierarchical hydroxyapatite formation, and d) demonstrating proof-of-concept by assessing scaffold performance using a rabbit model. This project pushes the boundaries of both 3D printing technology and bioactive bone scaffolds by engineering precision with a highly bioactive material and creating a self-healing osteoinductive/osteoconductive calcium phosphate 3D printed scaffold without the use of biologics for fast clinical translation.
-
AO CMF-17-24D Project title: Periosteal CAD-CAM prefabricated vascularized bone graftsOne of the foremost challenges in oral and maxillofacial surgery is the long-term reconstruction and prosthetic rehabilitation of patients presenting severely atrophied alveolar ridges or even large bone defects of the facial skeleton. Despite drawbacks, such as donor site morbidity and limited availability, the gold standard treatment remains the use of autologous bone grafts. Bone tissue engineering, often combined with cells and growth factors, developed into a promising alternative, aiming for tailor-made scaffolds fit to defect dimensions. On the other hand it has been shown that feasible volumes of bone can be engineered when utilizing an autologous artificial space (bioreactor) between compact bone and its periosteum. Still, the biggest challenges of this approach are 1.) locating a feasible and accessible area to create and manipulate this artificial space without causing a major secondary surgery site and subsequently donor site morbidity, and 2.) to create a bioreactor that provides a patient/ defect specific bone graft. In addition, early neovascularization needed to provide adequate oxygenation, preventing hypoxia, cell death and implant failure, is a prerequisite.
Consequently, the aim of our research is to evaluate the neovascularization potential and bone forming capacity of a CAD-CAM bioreactor located between the medial femur condyle and its periosteum. The location is determined by the defined periosteal blood supply of the descending genicular vessel that facilitates the design of a defined vascular pedicle flap, so that the construct will be located between the vascular pedicle flap (top) and the deperiosted compacta of the medial femur condyle (bottom).The bioreactor itself is composed of a CAD-CAM designed artificial PEEK construct comprising the medial, lateral, anterior and posterior wall of the bioreactor. The dimension of the bioreactor can be CAD-CAM designed according to the needed dimension. The bioreactor will be loaded with a beta tricalciumphosphate scaffold. In this study we intend to establish a CAD-CAM designed vascularized hybrid bone graft made of a scaffold and the adjacent pedicled periosteum within 6 weeks. The bone graft will be transferred and anastomosed to a critical sized defect created in the mandibular region and healing will be assessed for 8 weeks. Healing this defect using this periosteal CAD-CAM prefabricated vascularized hybrid bone graft will be compared with treatment using scaffold material alone