AO CMF Preclinical Research Projects
Computer-assisted ranking to facilitate risk evaluation, diagnosis and treatment decision
Applicants: L Kamer, H Noser
Background
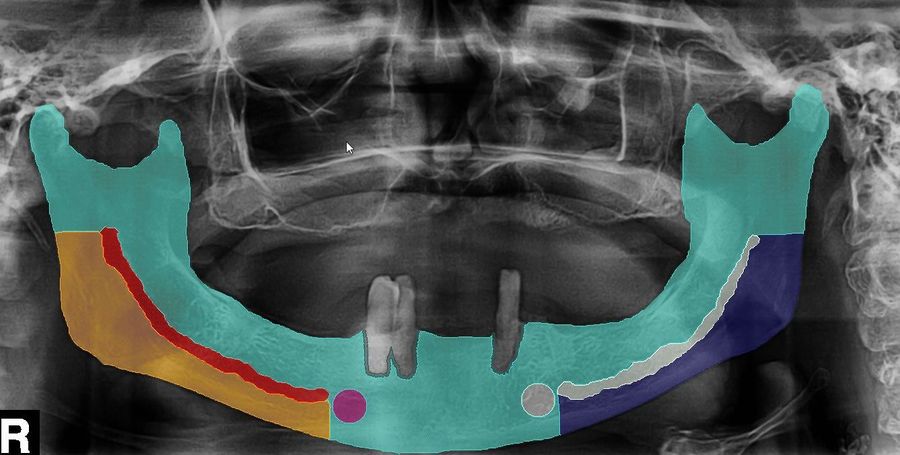
Antiresorptive drug-related osteonecrosis of the jaw (ARONJ) is a severe clinical condition that is related to management of bone diseases such as osteoporosis or cancer. Its early diagnosis and treatment may prevent or reduce the morbidity resulting from advanced destructive lesions of the jaws. Currently, diagnosis and treatment decisions are related to clinical tasks that usually rely on the medical history, clinical examination and radiographic assessment, as they are considered to be the most sensitive tools. However, in ARONJ, these tasks still remain difficult to be accomplished and leave a wide open space for subjective interpretation.Goal
To develop a computerized ARONJ ranking tool with implementation of Artificial Intelligence (AI) to rank given ARONJ cases in order to improve and facilitate the risk assessment, diagnosis and treatment decision in the clinics.Results
More than 200 patient records and image data (panoramic radiographs, computed tomography and cone beam computed tomography scans) were collected from a retrospective series of patients affected by ARONJ, by oral cancer with jaw bone involvement (reference group 1), as well as patients requiring dental implants (reference group 2). Labeling of the panoramic radiographs, computed tomography as well as cone beam computed tomography was performed. An AI workflow was developed that allowed for the data to be analysed within less than one minute.
Partners
- Rana M, Department for Oral and Maxillofacial Surgery, Heinrich Heine University of Düsseldorf, Düsseldorf, Germany
- Lutz I, Center of Dental, Oral and Maxillofacial Science, Medical School Hannover, Hannover Medical School, Hannover, Germany
- Gellrich NC and Zimmerer R, Department of Cranio-Maxillofacial Surgery, Hannover Medical School, Hannover, Germany
Printable Ceramic Ink for Patient Specific Implant Fabrication
Applicant: D Eglin, M Stoddart
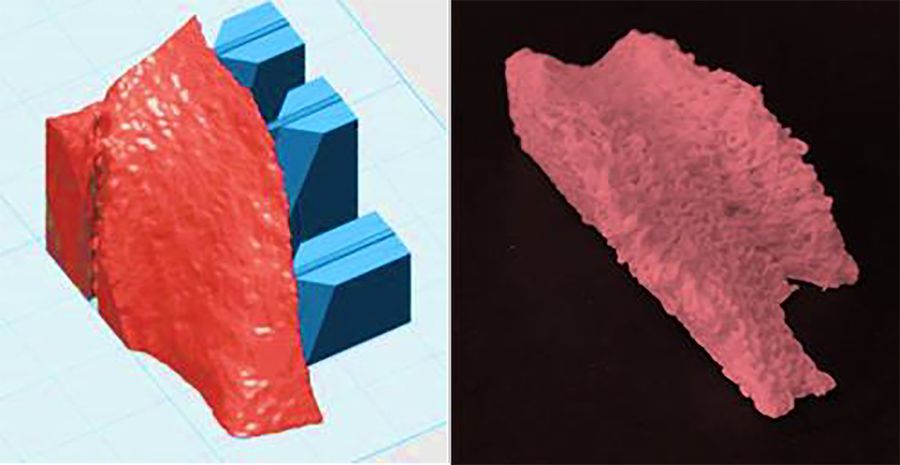
It is estimated that more than 500,000 bone-grafting procedures are performed annually in the US alone. Worldwide, the bone graft substitute market is expected to growth over 6 % in the next 10 years reaching over 5 billion dollars by the end of 2027. Among the different acute bone fractures leading to a high burden for our societies and unbearable affliction for the patient, the region of the head and the face is a major target for development of patient specific implants. Reconstruction of large bone fractures and defects in craniomaxillofacial surgery requires restitution of the pre-injury bone anatomy to re-establish form, function and aesthetics. To further improve the clinical outcome and to meet the criteria for a true-to-original reconstruction individual bone implant, new personalized printable materials are required.
The goal of this project is to optimize an osteoconductive calcium phosphate cement product for printing patient specific implants, focusing on the development of a fast printing and cement setting process for adequate handling, mechanical and structural stabilities and rapid implantation.
3D printing of cellularized tissue engineered constructs. Bioinks & methods toward clinical translation
Applicants: M Stoddart, D Eglin
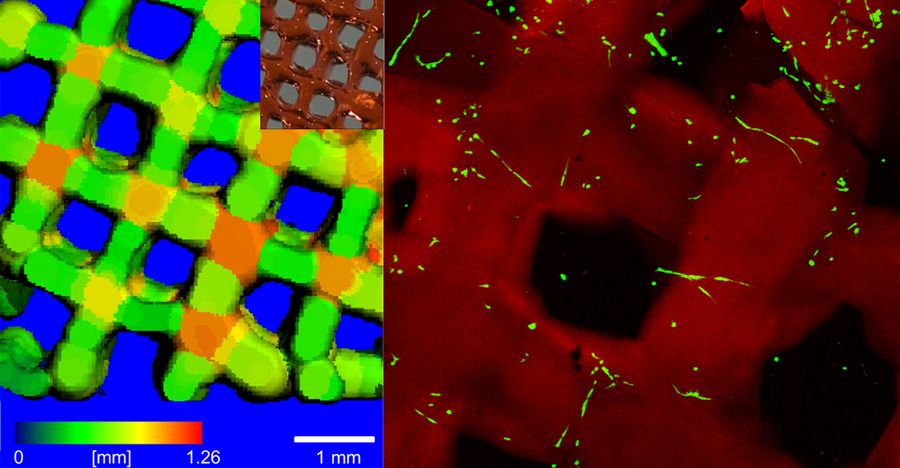
Patient specific implants based on additive manufacturing principles hold great promise in CMF applications, where anatomical fidelity is paramount. As the imaging to printing workflows improve, one major hurdle is the development of bioinks that are osteoinductive, while at the same time having a realistic path through regulatory approval. By avoiding complex material developments and following a "less is more" approach, we believe a novel material with clinical approval can be obtained faster. This project aims to investigate the printability of three clinically approved natural materials and further improve their function with minor modifications. The potential to improve their osteoinductive properties will be investigated by the addition of simple osteoinductive molecules that are already clinically approved or would have a less challenging approval process.
The goal of this project is to develop the 3D printing of clinically relevant biopolymer hydrogel products, namely collagen type I, fibrin glue and hyaluronan for the manufacturing of cellular 3D tissue engineered constructs.