Using fracture assessment to inform implant decision-making and reduction method
BY DR MARK GLYDE AND DR CHRIS TAN
This article describes a systematic method of applying biomechanical and biological assessments to ensure the most appropriate fracture fixation. Where anatomical reconstruction is indicated, it is performed because, with adequate fixation, it will allow load sharing while direct healing takes place. Where anatomical reconstruction is not achievable or only achievable with significant iatrogenic biologic damage, typical in comminuted fractures, a bridging fixator to span the gap or comminuted section and carry the full load is indicated.
Fracture type largely determines the indicated approach in non-articular fractures. Simple (non-comminuted) or minimally comminuted large fragment fractures are typically anatomically reconstructed as the biomechanical benefit outweighs the surgical biologic damage from direct fracture reduction. Comminuted fractures are typically bridged and axial and angular alignment achieved through indirect fracture reduction either closed, MIO or open-but-do-not-touch, as the biologic damage that is caused and the consequent delay in fracture healing is not justified by any biomechanical benefit.
Pre-operative planning saves time and patients
Only around 30% of veterinary orthopaedic complications are consequent to technical errors in the operating room. The rest result from poor decision-making and inadequate planning. That is, the vast majority of fracture fixation failures are consequent to temporal errors made prior to surgery rather than technical errors during surgery.
This article presents a series of steps to choose a fixation method that will provide the stability and durability necessary for each fracture type. It also provides a systematic method of evaluating the patient’s biological status and matching the result to a fixation system with the appropriate biomechanical characteristics.
Below we will cover:
- How to use radiographs to determine which fractures should be reconstructed
- How to use radiographs to determine contraindicated and indicated repair options
- How to explain the biomechanical benefit of anatomic reconstruction to achieve load-sharing
- How to explain the biological negatives of anatomic reconstruction
- How to perform a biomechanical fracture assessment— likely required implant “strength”
- How to perform a biological fracture assessment—likely healing time and appropriate repair options
- How to apply this knowledge to fracture decision-making and reduction method
How many limbs are involved?
Fracture fixation must withstand both repetitive or cyclic stresses from walking and running, and sudden major loads such as from jumping or sprinting. This is something largely unique to veterinary patients and not typically seen in human patients. Because damage to two or more legs will leave the animal unable to reduce the load on the fractured limb by limping, multiple limb injuries will require more robust fixation.
Which fractures should be reconstructed?
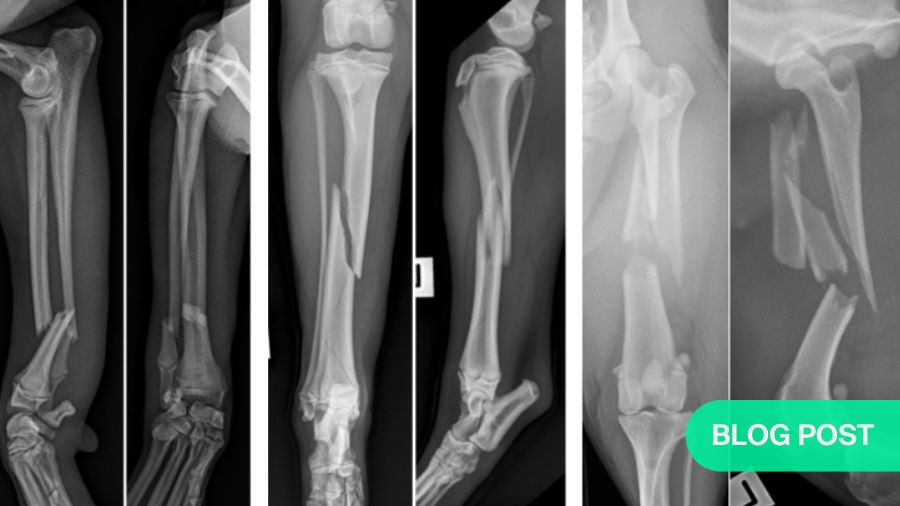
Clear radiographs are essential
For each fracture identified or suspected, it is a mandatory requirement to take two orthogonal views of the entire fracture bone including the proximal and distal joints —one craniocaudal (CC) or and one mediolateral (lateral view). It is also recommended to obtain orthogonal views of the intact opposite bone for the purpose of accurate fracture planning and implant templating.
Every repair comes at a cost
Once you have a clear view of the fracture and surrounding area, the first question to answer is, “Will an anatomic reconstruction be appropriate for this bone? Will the biomechanical benefits of direct fracture reductions and anatomic reconstruction outweigh the biologic damage that I do?” Alternatively if direct fracture reduction and anatomic reconstruction not achieve effective reconstruction of the bone column and thereby not allow load-sharing, then bridging fixation and minimisation of the biologic damage to the soft tissue / blood supply attachments to the comminuted fragments to speed fracture healing is preferable.
Examine your radiographs to determine contraindicated and indicated repair options
For simple fractures, anatomic reconstruction is normally indicated
Most importantly for fixation decisions, X-rays show how many bone fragments there are. If there are only two (a simple fracture), anatomic reconstruction is normally indicated. However, be sure to look closely for fissures in the bone, as these may indicate or lead to comminution and will weaken the bone.
Some very low-energy injuries, such as greenstick fractures, do not even require reconstruction, as they are already relatively stable, with little or no damage to the periosteum, the medullary artery or the surrounding muscle.
Blood flow to the bone is often severely restricted
In many cases, though, the fracture is complete and displaced. In addition to damaging the soft tissue envelope (the periosteum, surrounding muscle and connective tissue), this breaks the medullary artery—normally the bone’s main blood supply. Therefore, until the medullary blood flow resumes, the bone will rely entirely on the tiny periosteal arteries to feed the bone through muscle attachments. Therefore, care must be taken to minimize surgical damage to muscle attachments to the fractured bone.
Which part of the bone is fractured?
Because fractured areas depend on muscle attachments for their blood supply following fracture, fractures in locations with less muscle mass will heal more slowly. Using the tibia as an example, a fracture at the distal end will heal more slowly than one in the much more muscular proximal area.
Decisions with regard to strength of fixation should be made with consideration of healing time. Prolonged healing time in the absence of load-sharing, significantly increases the number of cycles or steps that fixation must withstand before development of fracture callus creates a load-sharing situation. Longer fracture healing duration requires stronger fixation to reduce the risk of cyclic or fatigue failure.
Four-piece (comminuted) fractures: anatomic reconstruction is not an option
A fracture with four or more pieces is far more complex than simple or non-comminuted two piece fractures. The presence of multiple bone fragments typically indicates high-energy trauma, such as a motor vehicle accident or gunshot injury. In addition to bone comminution, the surrounding soft tissue envelope, and therefore the blood supply to the fracture fragment, is often seriously damaged.
In such a case, anatomic reconstruction is almost always contraindicated as effective load-sharing can not be achieved and so the biomechanical benefit is not worth the biological “price” of iatrogenic surgical damage to the muscle attachments to the bone fragments. Even if the fragments can be pieced back together in their original configuration, effective load sharing will not be possible.
Exception: Joint fractures demand anatomic reconstruction or replacement
Any fracture that extends into a joint requires anatomic reduction at least of that part. If this is not feasible, prostheses are available, but outside the scope of this discussion.
In non-articular fractures, indirect healing through callus formation carries no inherent negative effect when compared to direct or non-callus healing. This is not the case for articular fractures.
Anatomic reconstruction with interfragmentary compression to achieve direct bone healing without callus is necessary. Healing with callus in a joint creates significant osteoarthritis and may significantly compromise joint function.
Three-piece injuries: the grey zone
Between the relatively straightforward decisions necessary for very simple and very complex cases lie three-piece fractures. For those where effective load-sharing can be achieved, anatomic reconstruction is a good investment. Otherwise, bridging fixation will be necessary. If in doubt, indirect fracture fixation and bridging fixation should be the default.
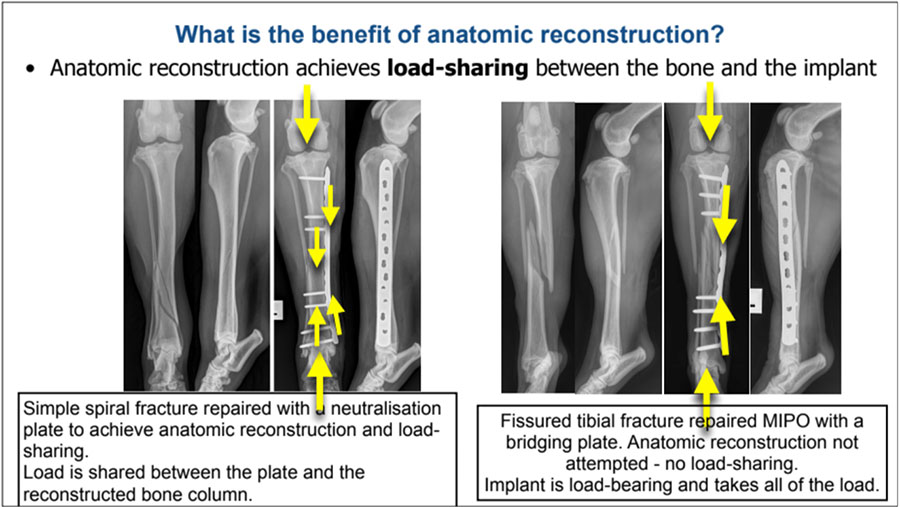
Explain the biomechanical benefit of anatomic reconstruction to achieve load-sharing
The intended biomechanical benefit of anatomic reconstruction is that the reconstructed bone column will share the load with the implant on weight-bearing while the bone heals. Anatomically reconstructed load-sharing constructs have high combined structural strength. This greatly reduces the risk of both catastrophic implant failure through acute overload, and of cyclic or fatigue failure.
Explain the biological negatives of anatomic reconstruction
For a more complex comminuted fracture, effective load-sharing is rarely achievable. In this case, incomplete or unstable reconstruction of the bone column not only fails to achieve biomechanical advantage, but does so at the cost of serious damage to the periosteal blood supply. This will slow healing and increase the risk of non-union. As heavyweight bridging fixation will still be necessary, a strategy that facilitates indirect healing will have a lower damage/benefit ratio.
Bridging options
Plating, interlocking nails, IM pins
In cases where load-sharing cannot be achieved, bridging fixation is necessary. As this spans gaps or comminuted segments, it must be very strong, stable, and durable enough to last the indirect healing period. Effective bridging fixation requires threaded connection of the fixation by screws or bolts or threaded pins to the bone. Options for threaded bridging fixation included bone plates, augmented plate fixation (plate-rod, orthogonal plates, double plating, dual bone fixation), interlocking nails and external fixation using threaded fixation pins.
No IM pins in the radius
In dogs, intramedullary (IM) pins can be used in all bones except the radius, as radiuspinning has a high complication rate exceeding 70%. The use of intramedullary pins in the radius is contraindicated.
External skeletal fixators
External skeletal fixators (ESF) are an effective form of bridging fixation, most suitable to lower limbs where placement of fixation pins in two planes along the length of the bone is achievable without penetrating muscle bundles. Placement of fixation pins through muscle mass will lead to pain for the patient, premature pin loosening, and an increased stand-off distance from the bone which decreases fixation stability. For this reason, placement of ESFs in the humerus or femur are usually better avoided. Internal fixation with bone plates or interlocking nails will have lower morbidity and achieve more effective stability in thse locations.
Additionally, healing time is a consideration for the use of ESFs. As a general rule, a well-placed threaded ESF, placed to avoid muscle mass, will last with effective stability and minimal complications in up to 12 weeks. Consideration should be given to the use of plates or interlocking nails if healing time is expected to exceed 12 weeks.
Biological fracture assessment—likely healing time and appropriate repair options
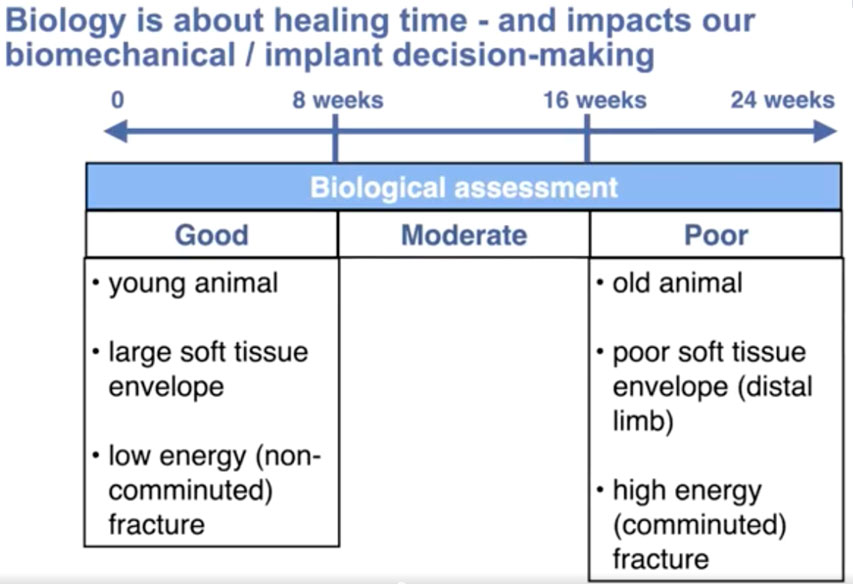
For our purposes, biological status is categorized by healing time. Virtually all small animal fractures in dogs and cats heal within 6 months. To simplify biological fracture assessment, rather than a numerical system, we can divide each patient’s overall biologic assessment / (healing time) into three categories: good (up to eight weeks), moderate (eight to 16 weeks) and poor (16 to 24 weeks).
The five most influential factors regarding healing time are:
- age;
- the blood supply to the fracture—particularly the condition of the soft tissue envelope—
a. before the fracture, with more distal fractures with a smaller pre-existing soft tissue envelope healing more slowly
b. after the fracture, with comminuted fractures having a more compromised extraosseous blood supply than simple fractures
c. after fixation; with direct open fracture reduction and anatomic reconstruction creating more iatrogenic soft tissue damage than indirect or closed reduction - fracture gap that creates a situation of high interfragmentary (IF) strain, with minimally stable small gap fractures experiencing the highest IF strain
- infection; or other bone disease
- any concurrent injuries or systemic diseases.
Of these, blood flow to the fracture site is the factor we have to understand the best to estimate healing time. Remember, in virtually every case of complete fracture, the medullary artery is ruptured and typically takes several weeks to repair. During this time, the blood supply to the fracture fragments comes almost exclusively from extraosseous sources though periosteal vessels connected to the bone through muscle attachments So, the size, extent condition of the soft tissue envelope is critical to the healing time.
While a low-energy injury will normally involve some soft tissue damage, the amount of energy transmitted into that tissue is low and so the damage to the blood supply is limited. For highly comminuted or high-energy fractures, the amount of energy transmitted instantaneously on bone fracture to the surrounding muscle is large, typically creating significant damage to the muscle attachments and thereby to the extraosseous blood supply.
In cases such as this, it is essential to minimize the “hit by surgeon” damage we do during the reduction and fracture repair. As the table below indicates, a high energy, comminuted fracture such as this often require six full months of healing time. Also, considering that there is no possibility of load-sharing—and therefore, no benefit to be had from an anatomic reduction—heavyweight bridging fixation will be necessary to withstand full load-baring and typically prolonged healing times.
Choosing an implant with the appropriate “Strength”: Biomechanical fracture assessment
When an implant fails, it is often tempting to blame faulty implants, excessive activity by the animal, or poor compliance by the owner in their care of their pet. In the vast majority of cases however, failure of fracture healing results from primarily decision-making errors where the vet chose fixation that lacked the strength and/or the durability to withstand the loads applied for the duration or number of cycles required.
Orthopaedic implants are engineered and manufactured to exacting specifications. When used appropriately, failures are uncommon. Small animal veterinary patients come in all shapes and sizes.
Dogs alone have roughly a 90-100 fold variation in weight. Even between two dogs of the same weight, factors such as age, fracture type and location, number of limbs fractured and expected activity level can lead to major differences regarding implant needs. Therefore, choosing the appropriate fixation for each case demands a realistic biological, biomechanical and clinical assessment.
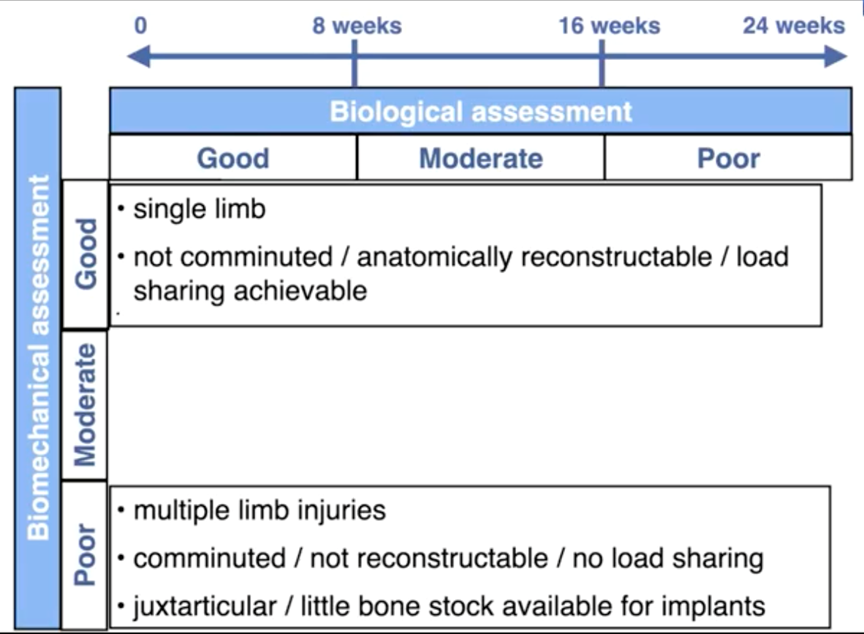
Five main considerations affect the stability of the repaired fracture:
- the type of fracture (incomplete or greenstick, simple, comminuted);
- whether the fracture can be anatomically reconstructed, i.e., whether load-sharing can be achieved;
- the method of fracture repair;
- whether it is a single- or multiple-limb injury; and
- the patient size and expected level of activity during healing.
Among “lightweight” fixation choices, appropriately applied casts, splints or slings can be expected to last reliably with a low likelihood of complications up to four weeks. IM pins with cerclage wire, again if appropriately applied, will typically last reliably with a low likelihood of complications, for up to 12 weeks.
Bone plates, interlocking nails and external skeletal fixators with threaded pins* can be considered “heavyweight” fixation options due to their threaded connection to the bone. Plates or augmented bone plates (plate-rod, orthogonal plates, dual bone fixation and double plating) and interlocking nails, if appropriately applied, can be expected to last a healing time of 6 months. Well applied threaded ESFs can typically be relied on to provide medium term stable fixation without complications for up to 12 weeks.
(* Smooth pins are available but not recommended, as they have only about half the working life of threaded ones.)
Combining biomechanical factors and biological factors
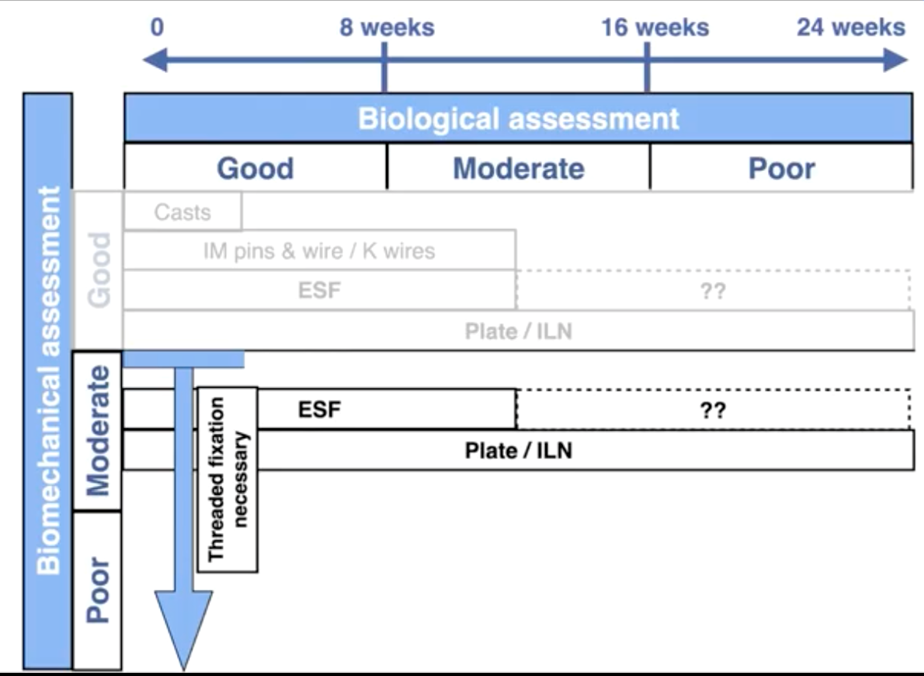
As shown below, by placing the three categories of biologic assessment / healing time as the x-axis of a chart, and the biomechanical assessment / required strength on the y-axis, it is possible to build a simple decision-making grid.
As this chart indicates, casts only provide appropriate fixation in fast healing fractures of the radius, tibia or distal limbs with good biomechanical assessment. An example would be an immature dog with a greenstick radius or tibial fracture.
IM pins are only suitable for simple long oblique or spiral fractures of the humerus, femur or tibia with good biology / fast healing and with a good biomechanical assessment.
External fixators are indicated for ideal use in the radius or tibia where good biological assessment indicates a healing time <12 weeks, yet provided they are placed with threaded fixation pins, are suitable for good to poor biomechanical assessments.
Where biological assessment indicates that healing time is likely to exceed 12 weeks, the use of plates or interlocking nails is advisable.
Apply this knowledge to fracture and reduction method decisions
The AO VET webinar on ‘Fundamentals of Fracture Decision Making—Using Fracture Assessment to Inform Implant Decision-making and Reduction Method’ includes three illustrative case studies to apply the information interactively.
Watch the recording with Mark Glyde and Christopher Tan:
About the authors:
Chris Tan is a specialist in small animal surgery based in Sydney, Australia. As an elected member of the Asia Pacific board of AO VET, Tan represents the region on the AO VET Education Commission. He conducts his research in collaboration with the Surgical and Orthopaedic Research Laboratories (SORL), University of New South Wales.
Mark Glyde is associate professor of small animal surgery at Murdoch University in Perth, Australia. As an AO member since 1990, he has served AO in a variety of leadership positions. His international reputation as a specialist clinician, educator, and researcher is reflected in 40 peer-reviewed publications in surgery and adult medical education as well as in his presentations at major international congresses.
You might also be interested in:
Lameness in dogs: diagnosis and decision-making
The 6-week AO VET online competency-based course focuses on enhancing decision-making skills and improving outcomes in canine surgery.

AO VET courses and events
Explore our upcoming courses, webinars, and online events in your region.
AO VET Guest Blog
Read more guest articles from the AO VET or submit your own posts to promote your work and passions.
How to get involved in AO VET
Becoming an AO VET friend could lead to a life-long partnership, from finding your best learning pathway to becoming an active officer.