Periprosthetic joint infection in 2025—genome, microbiome, and beyond
The human microbiome plays a crucial role in shaping human’s long-term health by modulating immune responses and protecting against infection. Disruptions in these microbial communities, known as dysbiosis, can increase susceptibility to infections, including postsurgical infections. In this third part of the series, Mustafa Citak from Helios ENDO-Klinik Hamburg, Hamburg, Germany, and Armita Armina Abedi from the Department of Orthopaedic Surgery and Traumatology, Copenhagen University Hospital, Bispebjerg, and the Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Denmark, will share current knowledge on the role of the human microbiome in surgical-site infections (SSIs) and periprosthetic joint infections (PJIs), with a focus on gut microbiome dysregulation, endogenous microbial sources of infection, and emerging strategies for prevention and patient optimization.

Mustafa Citak
Helios ENDO-Klinik Hamburg, Hamburg, Germany

Armita Armina Abedi
Department of Orthopaedic Surgery and Traumatology, Copenhagen University Hospital, Bispebjerg; Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Denmark
A look from within—the emerging role of the microbiome in joint infection
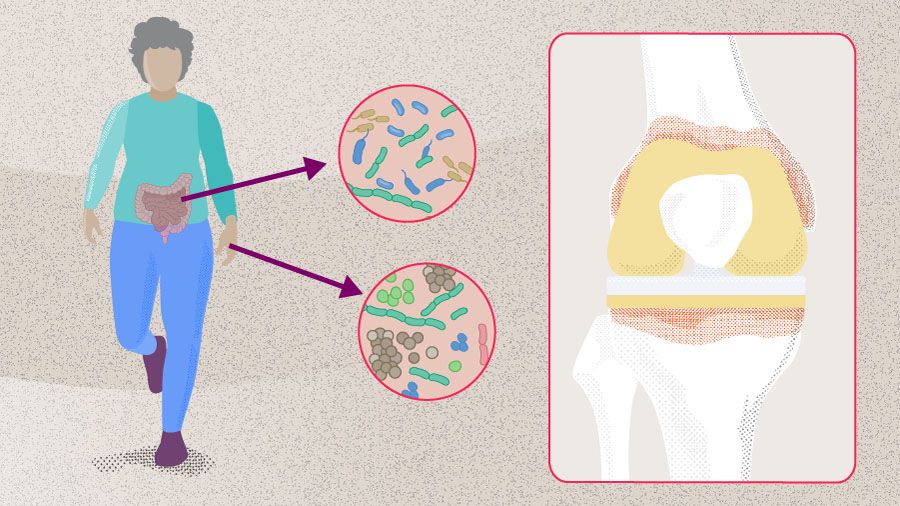
Total joint arthroplasty (TJA) is a safe and effective procedure that restores function and improves quality of life in patients with end-stage osteoarthritis (OA) [1]. However, its success can be undermined by PJI, a type of SSI that occurs in 1–2% of primary total joint replacement cases [2-4]. Periprosthetic joint infection occurs when microorganisms adhere to the implant surface and form biofilms that are resistant to host immune responses and antibiotics [5], leading to prolonged hospitalization, higher readmission rates, and increased morbidity and mortality [6].
Despite advancements in operative techniques, implant manufacturing, and sterilization protocols (see Part 1 on PJI prevention), the prevalence of PJI continues to rise, largely due to the increasing number of TJA procedures performed annually [7, 8]. While most research has focused on minimizing exogenous sources of pathogens at the time of surgery, emerging evidence suggests that both SSI and PJI can originate from endogenous sources of microorganisms—particularly from the gastrointestinal (GI) tract in the context of dysbiosis and impaired gut permeability. This highlights the potential role of the human microbiome as a risk factor [9].
The human microbiome
The human microbiome refers to the community of microorganisms that reside in and on our body, along with their collective genomes. It includes several species of bacteria, fungi, and viruses, with bacteria being the most abundant [10]. Evidence that microorganisms are part of the normal human system dates back to the late 19th century, when Theodor Escherich identified Escherichia coli in children’s intestinal flora and later studied the role of microbes in digestion and intestinal disease [11, 12]. Intensive study of the human microbiome began in the early 21st century and intensified after 2007 with the Human Microbiome Project (HMP), a 5-year large-scale collaborative effort to characterize microbial communities in the oral, nasal, vaginal, gut, and skin of healthy adults using 16S ribosomal RNA (rRNA) gene sequencing [10]. The HMP also included demonstration studies of disorders such as psoriasis and ulcerative colitis, revealing major differences in microbial composition between individuals and across body sites in a single individual; this supported the theory that different body sites are host to a “core microbiome” [9, 10]. Over the last decade, advances in high-throughput sequencing and computational methods have greatly improved our understanding of the human microbiota composition, function, and its role in physiology and disease [6]. Emerging evidence suggests that microbial composition varies in different areas of the body more than previously recognized. This knowledge is becoming increasingly relevant in orthopedic surgery, as recent studies implicate dysregulation of the skin and the gut microbiome as potential sources of SSI and PJI through mechanisms such as bacterial translocation or impaired immune responses [9]. In this article, we review the link between the microbiome, SSI, and PJI.
Contribution of the patient skin microbiome to surgical-site infection
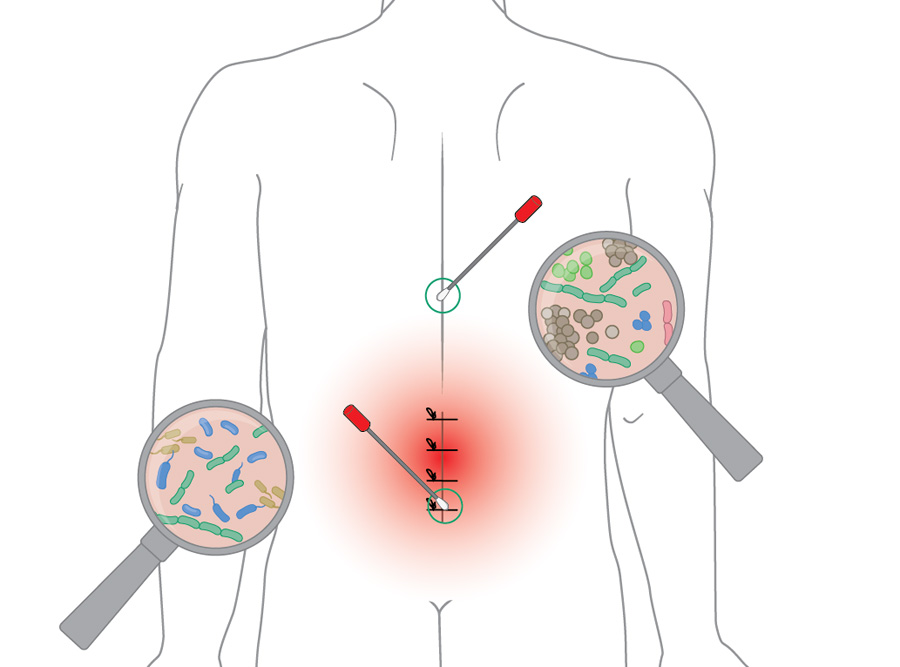
Surgical-site infection is a frequent postoperative complication, with a global incidence of about 2.5% [13]. The skin microbiome plays a key role in SSI development, as resident microorganisms often serve as the primary source of pathogens introduced during surgery. A recent groundbreaking study by Long et al [14] provides compelling evidence that many pathogens causing SSIs in instrumented spine surgery originate from endogenous rather than exogenous sources—that is, from the patients’ own microbiomes. They collected preoperative skin swabs from multiple anatomical regions along the back in a cohort of 124 patients undergoing posterior spinal fusion to assess variation in microbiome composition. The analysis revealed an anatomical cervicolumbar gradient along the skin of the patients, with microbial communities shifting from predominantly Gram-positive bacteria in the cervical and thoracic regions to more Gram-negative and anaerobic organisms (eg, Escherichia, Enterobacter, Bacteroides) in the lumbosacral area (Figure 1). Importantly, this microbial gradient mirrored the pathogens isolated in postoperative SSI, highlighting the importance of site-specific microbial patterns in influencing infection risk and suggesting potential implications for tailored antibiotic prophylaxis.
In the same study, Long et al [14] compared microbes isolated from SSI with preoperative endogenous strains obtained from the patients’ skin microbiome. They used genome capture sequencing (GenCap-Seq, see “Did you know” for method details) to analyze preoperative nasal, rectal, and skin swabs, along with postoperative SSI isolates, in a prospective cohort of 204 patients. In 86% of SSIs, the causative pathogens matched strains present in the patient’s preoperative microbiome, demonstrating that most infections are endogenous rather than exogenous. Furthermore, 59% of SSI pathogens were resistant to the prophylactic antibiotics given during surgery, corresponding to resistance genes already present in the patient microbiome. These findings provide robust data to demonstrate that many pathogens causing SSIs originate from the patient’s own microbiome and preexisting resistance, suggesting that individualized preoperative microbiome profiling could guide personalized perioperative antibiotic strategies and challenge the traditional assumption that external contamination is the main driver of SSI.
As Abedi points out, “This study is groundbreaking because it shows that a patient’s own endogenous microbiome plays a key role in the risk of SSI, and in fact, in the study, all the microbes in the postoperative isolates were already present in the patients beforehand. From a prevention perspective, this really shifts attention toward strategies aimed at reducing the patient’s own microbial burden and recognizing the growing importance of the microbiome in surgical outcomes.” She adds, “Another interesting finding is that many of the bacteria identified were already resistant to the antibiotics given as standard prophylaxis. That really highlights the need for personalized infection-prevention strategies that take each patient’s preoperative microbiome into account”.
Did you know?
Genome capture sequencing (GenCap-Seq), first described by Hayden et al (2022) [15], is a low-cost, scalable method that uses custom-made hybridization probes to enrich bacterial DNA from complex human samples such as skin, sputum and feces, enabling genome-wide measurements of bacterial allelic diversity, providing a low-cost method for the analysis of preoperative (metagenomic) and postoperative (SSI-isolate) pairs.
The emerging concept of the “joint microbiome”
Recent research has also introduced the concept of a joint microbiome—referring to the community of microorganisms residing within the joint space—challenging the long-held belief that joints are sterile environments [16]. Key findings from various studies have identified diverse microbial populations in the synovial fluid and tissues of patients with OA, suggesting these microorganisms may contribute to disease pathogenesis [17]. In a recent prospective study, Fernandez et al [16] investigated how microbial profiles differ across knee conditions. Synovial fluid from 65 knees (in 55 patients)—including normal control, OA, aseptic revision, revision for PJI, and contralateral knees in PJI patients—was analyzed using both traditional culture and next-generation sequencing (NGS). The study revealed greater bacterial diversity in the knees of patients with OA, particularly enriched with Proteobacteria (new Pseudomonadota), a major component of the gut microbiome. In contrast, nearly 75% of bacterial species in healthy controls consisted of Cutibacterium, Staphylococcus, and Paracoccus species. The authors concluded that OA and other knee conditions are associated with distinct knee microbiome profiles, suggesting that native microbial communities may contribute to joint disease pathogenesis and influence susceptibility to PJI [16].
Together with the work of Long et al [14], these findings highlight the presence of site-specific core microbiomes—whether within the skin or the joint—that may influence musculoskeletal disease processes and surgical outcomes.
The gut-joint axis—gut microbiome dysbiosis and its role in periprosthetic joint infection
The microbiome is a dynamic, intricate network of microbial species unique to each individual which begins colonization at birth and varies based on ethnicity, diet, and environment [6]. These microbes live on our skin, along the GI tract, at other mucosal surfaces, and sometimes even inside our cells. In the GI tract, Firmicutes (new Bacillota), Actinomycetota, and Proteobacteria dominate, making up over 90% of the microbial community [6, 18].
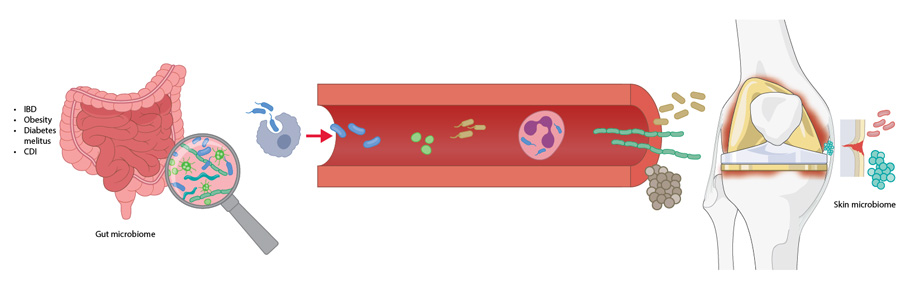
The components of a healthy gut microbiota form a stable community and their biochemical activities have a beneficial impact on the host, conferring resistance to nonnative bacteria, developing the host immune system, and exerting important metabolic functions [19]. It is generally accepted that changes in the relative abundance of the predominant species within the core microbiome have consequences for human health. This is termed dysbiosis and is defined as a disruption of the core microbiome through loss of beneficial organisms, overgrowth of harmful ones, or reduced diversity [20]. Dysbiosis may arise from prolonged antibiotic use or unhealthy dietary habits, both of which disrupt normal gut physiology, and has been linked to conditions such as obesity, diabetes, inflammatory bowel disease (IBD), and Clostridium difficile (new Clostridioides difficile) infection (CDI). These conditions may, in turn, contribute to SSI and PJI through compromised gut barrier function, increased gut permeability, and mechanisms such as the “Trojan Horse hypothesis”, which will be discussed in the following sections [21]. Together, these insights have given rise to the concept of the gut–immune–joint axis—the dynamic crosstalk between the gut microbiome and the joint environment—highlighting how systemic dysbiosis can drive joint infection and inflammation.
Inflammatory bowel disease, gut permeability, and the “Trojan Horse Hypothesis”
Inflammatory bowel disease affects millions worldwide and has shown a rising incidence over the past 50 years [22]. Gut dysbiosis is known to occur in patients with IBD and has been linked to an increased risk of PJI [22]. In a matched cohort study of 152 IBD patients and 146 controls undergoing primary TJA, Chisari et al [22] reported a significantly higher cumulative incidence of PJI in the IBD group (4.61% vs 0.88%; P = .0024), with IBD emerging as an independent risk factor (HR 5.44). Inflammatory bowel disease was also linked to higher rates of aseptic revision and postoperative complications. Importantly, patients with IBD in this study were not receiving immunosuppressive therapy, which is itself a well-established risk factor for PJI. The authors proposed the “Trojan Horse Hypothesis” mechanism, whereby gut-resident bacteria such as Staphylococcus aureus may translocate to distant sites not only through the blood (ie, bacteremia), but also via immune cells like macrophages and neutrophils [21]. These cells act as carriers, transporting internalized bacteria to distant sites and potentially contributing to PJI. Additionally, increased gut permeability exacerbates this issue, allowing microbial products such as lipopolysaccharides (LPS for PJI) to enter systemic circulation. Elevated LPS levels can induce chronic low-grade inflammation, characterized by heightened IL-6 and TNF-α levels, which impair perioperative immune function at surgical sites [21]. Although evidence for the Trojan horse hypothesis is currently limited to preclinical murine models—where Staphylococcus aureus has been shown to translocate from the gut to the surgical site [23, 24]—this mechanism may partly explain the high infection rate observed in patients with IBD, and it provides a compelling model for how endogenous microbes could seed prosthetic joints.
Future clinical studies are needed to validate this hypothesis in humans, but indirect mechanistic evidence linking gut permeability to PJI has already been presented by the same group [25]. In a prospective study examining 134 patients undergoing primary arthroplasty or revision arthroplasty because of an aseptic failure or PJI (44 PJI: 30 chronic, 14 acute; 90 noninfected controls: 64 patients revised for aseptic failure, 26 patients undergoing TJA) [25], they found that plasma levels of the gut permeability markers zonulin and soluble CD14 (sCD14) were significantly higher in PJI patients compared to those undergoing aseptic revision (zonulin: 7.64 ± 6.08 vs 4.56 ± 3.83 ng/mL, P < 0.001; sCD14: 555.72 ± 216.66 vs. 396.87 ± 247.92 ng/mL, P = 0.003). Additionally, acute infections exhibited higher zonulin levels than chronic cases (11.60 ± 6.72 vs. 5.80 ± 4.84 ng/mL, P = 0.005). These findings link impaired gut barrier function to increased PJI risk and suggest that gut-targeted interventions with modulators of the gastrointestinal tract could potentially complement antibiotic therapy.
Body mass index and diabetes as risk factors for periprosthetic joint infection
Body mass index (BMI) is a well-established risk factor for PJI, with multiple studies reporting higher infection rates in obese patients [26, 27]. However, it remains unclear whether microbial profiles differ among PJI patients with varying BMI. Budin et al [28] addressed this question by investigating the relationship between BMI and microbial profiles in patients with PJI following total hip arthroplasty (THA). In a retrospective cohort study of 3,645 PJI cases after THA, they identified distinct microbial profiles associated with different BMI groups. Patients with BMI ≥ 40 had higher odds of infection by specific pathogens: Streptococcus dysgalactiae (odds ratio [OR] ~ 9.9), Proteus mirabilis (OR ~7.4), and Klebsiella pneumoniae (OR ~6.9), as well as more frequent polymicrobial PJIs (OR ~ 2.2), defined as infections involving multiple organisms simultaneously colonizing a single prosthesis. Increasing BMI was associated with altered microbial balance, characterized by a predominance of Firmicutes (eg, Enterococcus faecalis, Enterococcus species [spp.], Staphylococcus epidermidis, Staphylococcus haemolyticus, Staphylococcus hominis, and Streptotoccus dysgalactiae) and Proteobacteria (eg, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, and Pseudomonas aeruginosa) in the gastrointestinal microbiome of obese patients. Furthermore, the study highlighted several comorbidities including diabetes mellitus, hyperlipidaemia, and hypertension, suggesting an underlying metabolic syndrome potentially contributing to the “leaky gut syndrome”, a condition in which disruption of the gut barrier integrity allows microorganisms and dietary components to enter the bloodstream, facilitating bacterial translocation from the gut to the surgical site [29].
Microbial diversity has been explored also in the context of diabetes, as diabetic patients are known to have higher rates of postoperative infections, including PJIs, compared to nondiabetic patients [30, 31]. To explore microbial diversity in this context, Engin et al [32] conducted a large retrospective cohort study of 4,261 hip arthroplasty PJIs (1996–2021). While Staphylococcus spp. predominated in both diabetic and nondiabetic patients, diabetes was associated with significantly higher odds of infection by Candida albicans (OR 2.8), Klebsiella pneumoniae (OR 2.4), Staphylococcus aureus (OR 1.3), Staphylococcus epidermidis (OR 1.7), Clostridium perfringens (OR 5.9), and polymicrobial infections (OR 1.5). Notably, polymicrobial PJIs are associated with worse outcomes than monomicrobial cases. These findings suggest that diabetes predisposes patients to a broader and more diverse spectrum of infective organisms, likely due to factors such as hyperglycemia-induced immune dysfunction, impaired tissue integrity, and enhanced microbial virulence [32].
As Citak notes, “BMI and diabetes often go hand in hand—patients with high BMI frequently also have diabetes. Together, these conditions likely represent the highest risk for complications, not only infections but also aseptic loosening. In recent years, as more research has been published, there has been growing attention to prevention, especially in specialized centers. At Helios Endo-Klinik, for example, we have established clear thresholds for delaying elective surgery until patient optimization: a BMI cutoff of 40 and an HbA1c cutoff of 8.0% (indicating hyperglycemia). The rationale is simple—performing surgery without optimization may expose patients to avoidable infection risks without delivering meaningful benefits. Instead, we focus on patient optimization before surgery to improve outcomes. This includes encouraging weight loss, increasingly supported by medical therapies such as semaglutide-related medications, which have proven effective in helping patients lose weight before surgery.”
Clostridium difficile infection
Another condition that is considered pathognomonic for gut dysbiosis is CDI. Clostridium difficile is an anaerobic, Gram-positive, and endospore-forming bacterium and a leading cause of healthcare-associated postantibiotic colitis worldwide [33]. While it can asymptomatically colonize the intestines of healthy people, it might become virulent in the context of gut dysbiosis. A CDI is typically the result of perturbations in gut microbiome composition, mainly due to the use of antibiotics. Verhey et al [34] sought to determine whether a prior CDI increased the risk of PJI after total knee arthroplasty (TKA). Using a large patient claims database, they identified 5,170 patients with a history of CDI in the 2 years prior to TKA and matched them to controls. A prior CDI was independently associated with more than double the odds of PJI (OR 2.1; 95% confidence interval [CI]: 1.91–2.36), with the highest risk seen when CDI occurred within 3 months of surgery (OR 4.19). Patients with CDI in the 2 years prior to TKA were also far more likely to experience recurrent CDI after surgery (OR 25.9). Similarly, Deckey et al [35] found that CDI within 2 years of THA independently increased the risk of PJI, with the highest risk when CDI occurred within 3 months of surgery. These findings strengthen the link between gut dysbiosis and postoperative complications, identifying CDI as a time-sensitive, independent risk factor for PJI. They also highlighted the importance of assessing the patient CDI history before arthroplasty and, when feasible, delaying elective TKA for at least 1 year after CDI to reduce infection risk.
Conclusion
Surgical-site infection and PJI remain among the most serious complications after joint replacement, and growing evidence challenges the traditional view that external contamination is the primary source of infection, highlighting the patient’s own microbiome as a critical contributor [6, 9, 36]. Advances in sequencing technologies have revealed site-specific microbial communities in the skin and within the joint itself, and the elegant study by Long et al [14] compels surgeons to consider endogenous sources in SSI pathogenesis and suggests that individualized preoperative microbiome profiling could guide personalized perioperative antibiotic strategies.
We have also seen that gut dysbiosis is a key driver of infection risk, with several patient-related factors—including diabetes, obesity, CDI, and IBD—that contribute to this risk and should be carefully evaluated before TJA (Figure 2). Prevention strategies should focus on mitigating these risks through weight loss, optimization of glycemic control, and through assessment of CDI history in surgical candidates. Additionally, future research should prioritize the development of reliable biomarkers for defining gut dysbiosis [36].
Finally, emerging orthopedic research—including a recent publication [36] and findings presented at the 3rd International Consensus Meeting (ICM) in Istanbul, Turkey [37, 38]—suggests that preserving gut microbiota integrity may help mitigate systemic inflammation and infection risk, highlighting the potential role of probiotics as adjunctive therapy in PJI prevention [38] [36]. Preclinical studies indicate that probiotics can reduce systemic inflammation and enhance local immune responses; although clinical data specific to PJI remains limited, studies in broader surgical contexts provide some evidence of the potential benefits of probiotics. Several ongoing clinical trials are now investigating probiotics, prebiotics, and microbiome-informed strategies to reduce the risk of SSI and PJI. Moving forward, integrating the microbiome into perioperative risk assessment and infection prevention may represent the next frontier in arthroplasty [37].
AO Recon resources
Contributing experts

Armita Armina Abedi
Department of Orthopaedic Surgery and Traumatology, Copenhagen University Hospital, Bispebjerg
Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Denmark

Kristen Barton
Midwest Orthopaedics at Rush, Rush University Medical Centre, Chicago
Department of Physical Therapy, Western University, London, Canada

Mustafa Citak
Helios ENDO-Klinik Hamburg, Hamburg, Germany

Jason Jennings
Colorado Joint Replacement, AdventHealth, Denver, Colorado
Department of Mechanical and Materials Engineering, University of Denver, Denver, Colorado
Department of Physical Therapy, University of Delaware, Newark, Delaware
This article was written by Chiara Cianciolo, AO Innovation Translation Center, Clinical Science, Switzerland.
References
-
Pulido L, Ghanem E, Joshi A, et al. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008 Jul;466(7):1710-1715.
-
Ahmed SS, Haddad FS. Prosthetic joint infection. Bone Joint Res. 2019 Nov;8(11):570-572.
-
Ong KL, Kurtz SM, Lau E, et al. Prosthetic joint infection risk after total hip arthroplasty in the Medicare population. J Arthroplasty. 2009 Sep;24(6 Suppl):105-109.
-
Corvec S, Portillo ME, Pasticci BM, et al. Epidemiology and new developments in the diagnosis of prosthetic joint infection. Int J Artif Organs. 2012 Oct;35(10):923-934.
-
Izakovicova P, Borens O, Trampuz A. Periprosthetic joint infection: current concepts and outlook. EFORT Open Reviews. 2019 29 Jul. 2019;4(7):482-494.
-
Longo UG, Lalli A, Bandini B, et al. The influence of gut microbiome on periprosthetic joint infections: State-of-the art. Journal of ISAKOS. 2024 2024/06/01/;9(3):353-361.
-
Kurtz SM, Lau EC, Son M-S, et al. Are We Winning or Losing the Battle With Periprosthetic Joint Infection: Trends in Periprosthetic Joint Infection and Mortality Risk for the Medicare Population. The Journal of Arthroplasty. 2018;33(10):3238-3245.
-
He M-C, Ferrini A, Parvizi J. Periprosthetic joint infection: Time to think outside the box. Knee Surgery, Sports Traumatology, Arthroscopy.n/a(n/a).
-
Heckmann ND, Culler MW, Mont MA, et al. Emerging Concepts in Periprosthetic Joint Infection Research: The Human Microbiome. J Arthroplasty. 2025 Jul;40(7):1821-1826.
-
Turnbaugh PJ, Ley RE, Hamady M, et al. The Human Microbiome Project. Nature. 2007 2007/10/01;449(7164):804-810.
-
Escherich T. Die darmbakterien des säuglings und ihre beziehungen zur physiologie der Verdauung: Enke; 1886.
-
Escherich T. Die Darmbacterien des Neugeborenen und Säuglings. Fortschritte der Medicin. 1885;3(16 und 17):515-554.
-
Mengistu DA, Alemu A, Abdukadir AA, et al. Global Incidence of Surgical Site Infection Among Patients: Systematic Review and Meta-Analysis. Inquiry. 2023 Jan-Dec;60:469580231162549.
-
Long DR, Bryson-Cahn C, Waalkes A, et al. Contribution of the patient microbiome to surgical site infection and antibiotic prophylaxis failure in spine surgery. Sci Transl Med. 2024 Apr 10;16(742):eadk8222.
-
Hayden HS, Joshi S, Radey MC, et al. Genome Capture Sequencing Selectively Enriches Bacterial DNA and Enables Genome-Wide Measurement of Intrastrain Genetic Diversity in Human Infections. mBio. 2022 Oct 26;13(5):e0142422.
-
Fernández-Rodríguez D, Baker CM, Tarabichi S, et al. Mark Coventry Award: Human Knee Has a Distinct Microbiome: Implications for Periprosthetic Joint Infection. J Arthroplasty. 2023 Jun;38(6s):S2-s6.
-
He M, Kolhoff F, Mont MA, Parvizi J. Is Osteoarthritis a State of Joint Dysbiosis? Antibiotics. 2025;14(6):609.
-
Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature. 2011 2011/05/01;473(7346):174-180.
-
Gilbert JA, Blaser MJ, Caporaso JG, et al. Current understanding of the human microbiome. Nature Medicine. 2018 2018/04/01;24(4):392-400.
-
DeGruttola AK, Low D, Mizoguchi A, Mizoguchi E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm Bowel Dis. 2016 May;22(5):1137-1150.
-
Chisari E, Cho J, Wouthuyzen-Bakker M, Parvizi J. Periprosthetic Joint Infection and the Trojan Horse Theory: Examining the Role of Gut Dysbiosis and Epithelial Integrity. The Journal of Arthroplasty. 2022;37(7):1369-1374.
-
Chisari E, D'Mello D, Sherman MB, Parvizi J. Inflammatory Bowel Diseases Increase the Risk of Periprosthetic Joint Infection. J Bone Joint Surg Am. 2022 Jan 19;104(2):160-165.
-
Zhu H, Jin H, Zhang C, Yuan T. Intestinal methicillin-resistant Staphylococcus aureus causes prosthetic infection via 'Trojan Horse' mechanism: Evidence from a rat model. Bone Joint Res. 2020 Apr;9(4):152-161.
-
Krezalek MA, Hyoju S, Zaborin A, et al. Can Methicillin-resistant Staphylococcus aureus Silently Travel From the Gut to the Wound and Cause Postoperative Infection? Modeling the "Trojan Horse Hypothesis". Ann Surg. 2018 Apr;267(4):749-758.
-
Chisari E, Cho J, Wouthuyzen-Bakker M, Parvizi J. Gut permeability may be associated with periprosthetic joint infection after total hip and knee arthroplasty. Scientific Reports. 2022 2022/09/05;12(1):15094.
-
Wilson CJ, Georgiou KR, Oburu E, et al. Surgical site infection in overweight and obese Total Knee Arthroplasty patients. Journal of Orthopaedics. 2018 2018/06/01/;15(2):328-332.
-
Chaudhry H, Ponnusamy K, Somerville L, et al. Revision Rates and Functional Outcomes Among Severely, Morbidly, and Super-Obese Patients Following Primary Total Knee Arthroplasty: A Systematic Review and Meta-Analysis. JBJS Reviews. 2019;7(7).
-
Budin M, Sandiford NA, Gehrke T, Citak M. Body mass index matters: morbid obese patients have different microorganism profiles in the setting of periprosthetic hip joint infections. Int Orthop. 2025 Jun;49(6):1309-1317.
-
Camilleri M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. 2019;68(8):1516-1526.
-
Pedersen AB, Mehnert F, Johnsen SP, Sørensen HT. Risk of revision of a total hip replacement in patients with diabetes mellitus: a population-based follow up study. J Bone Joint Surg Br. 2010 Jul;92(7):929-934.
-
Maradit Kremers H, Lewallen LW, Mabry TM, et al. Diabetes Mellitus, Hyperglycemia, Hemoglobin A1C and the Risk of Prosthetic Joint Infections in Total Hip and Knee Arthroplasty. The Journal of Arthroplasty. 2015 2015/03/01/;30(3):439-443.
-
Ergin M, Budin M, Canbaz SB, et al. Microbial Diversity of Periprosthetic Joint Infections in Diabetic and Nondiabetic Patients Following Hip Arthroplasty. J Arthroplasty. 2025 Feb;40(2):494-498.
-
Mada PK, Alam MU. Clostridioides difficile infection. StatPearls. Treasure Island (FL): StatPearls Publishing
Copyright © 2025, StatPearls Publishing LLC.; 2025. -
Verhey JT, Boddu SP, Tarabichi S, et al. Gut-Joint Axis: History of Clostridium Difficile Infection Increases the Risk of Periprosthetic Joint Infection After Total Knee Arthroplasty. J Arthroplasty. 2025 Aug;40(8):2126-2130.
-
Deckey DG, Boddu SP, Verhey JT, et al. Clostridium difficile Infection Prior to Total Hip Arthroplasty Independently Increases the Risk of Periprosthetic Joint Infection. The Journal of Arthroplasty. 2024;39(9):S444-S448.e441.
-
Ramasamy B, Sharma DK, Callary SA, et al. Role of gut microbiota disruption in prosthetic joint infection: a scoping review. The Lancet Microbe.
-
Atipiboonsin V, Chisari E, Megaloikonomos PD, et al., editors. G25 – Does gut microbiome have a role in the development of Surgical Site Infection (SSI) and/or Periprosthetic Joint Infection (PJI) in patients undergoing major orthopedic surgery? The International Consensus Meeting on Infection (ICMI); 2025 8-10 May, 2025; Istambul2025.
-
Shahi A, Goswami K, Drago L, et al., editors. G26: Does the Use of Probiotic Agents Have Any Role in Preventing Surgical Site Infection (SSI)/Periprosthetic Joint Infection (PJI) After Major Orthopedic Surgery? The International Consensus Meeting on Infection (ICMI); 2025 8-10 May, 2025; Istambul2025.