Diagnosis of periprosthetic joint infection—what is the current thinking?
Diagnosing a periprosthetic joint infection (PJI) may be easy for a clinician when a patient presents with a purulent wound. But what about the patient with a chronic, more indolent infection? Periprosthetic joint infection presents in many ways, so how can it be best diagnosed? In this part of the series, Jason Jennings from the Department of Mechanical and Materials Engineering, University of Denver, Colorado, and the Department of Physical Therapy, University of Delaware, Delaware and Kristen Barton from Midwest Orthopaedics at Rush, Rush University Medical Centre, Chicago, and the Department of Physical Therapy, Western University, Canada, walk us through the known diagnostic criteria for PJI, explaining PJI testing, and the history of the guidelines, while giving us first-hand insights into the latest changes from the recently convened 2025 International Consensus Meeting (ICM).

Jason Jennings
Colorado Joint Replacement, AdventHealth, Denver, Colorado
Department of Mechanical and Materials Engineering, University of Denver, Denver, Colorado
Department of Physical Therapy, University of Delaware, Newark, Delaware

Kristen Barton
Midwest Orthopaedics at Rush, Rush University Medical Centre, Chicago
Department of Physical Therapy, Western University, London, Canada
Periprosthetic joint infection is a challenging clinical phenomenon, for which no gold standard diagnostic test is available [1, 2] and for which proposed definitions are closely linked to the fulfilment of PJI diagnostic criteria [1, 3–5]. Over the last several years, these diagnostic criteria have undergone multiple iterations from different groups and have achieved varying levels of acceptance in the clinical landscape [5]. Having a good understanding of how the guidelines have evolved and their differences may help the clinician better diagnose PJI by increasing awareness of the practicalities and subtleties of the diagnostic criteria. At the same time it also provides great insight into the challenges that a clinician may face when confronted with a patient with potential PJI, a potentially complex situation that may lead to invasive treatment with a risk of significant adverse events [5].
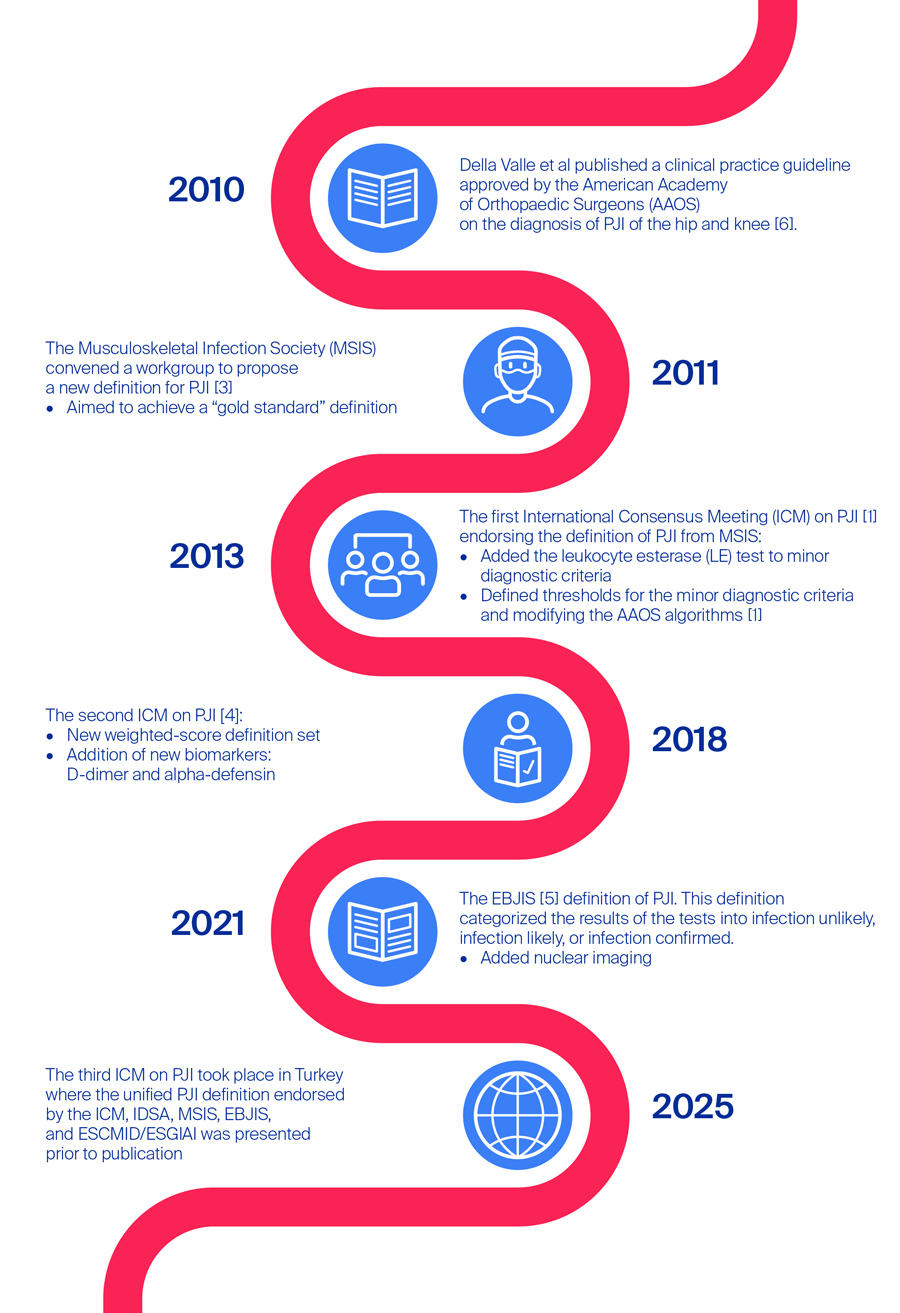
A brief history of the periprosthetic joint infection diagnostic guidelines
In 2010, Della Valle et al [6] published a clinical practice guideline approved by the American Academy of Orthopaedic Surgeons (AAOS) on the diagnosis of PJI of the hip and knee. The goal at the time was to improve PJI treatment based on the evidence available, and as a result the authors produced two practical diagnostic algorithms, one for patients with a high probability of hip or knee PJI and one for those with a low probability [6]. The authors also included 15 recommendations for the diagnosis of PJI [6]. The outgoing question for both algorithms was based on elevated erythrocyte sedimentation rate (ESR) or C-reactive protein level (CRP), followed by joint aspiration to assess cell count, differential, culture, and later frozen sections and/or intraoperative synovial fluid white blood cell (WBC) count and differential [6]. In high probability PJI patients, nuclear imaging was also recommended, with modalities including labeled leukocyte imaging combined with bone or bone marrow imaging, fluorine-18 fluorodeoxyglucose positron-emission tomography, gallium imaging, or labeled leukocyte imaging [6]. Around the same time, the Musculoskeletal Infection Society (MSIS), convened a workgroup to propose a new definition for PJI [3] aiming to achieve a “gold standard” definition. In addition to the two clear-cut major criteria (1) a sinus tract communicating with the prosthesis or (2) pathogen isolated by culture from at least two separate tissue or fluid samples from the affected prosthetic joint, there were six minor criteria based on serum and synovial tests as well as histology [3]. Similar to Della Valle et al’s AAOS guidelines [6], the first three of these minor criteria were (1) elevated serum ESR and CRP (where elevated pertained to an ESR of > 30 mm/h and a CRP of > 10 mg/L), followed by (2) elevated synovial leukocyte count and (3) elevated synovial neutrophil percentage (synovial leukocyte count of 1100–4000 cells/µL and polymorphonuclear neutrophils [PMN]% of 64–69%) [3]. It was noted in this guideline that, firstly, there is variance in laboratory tests that should be taken into account when performing serum tests, and secondly, the PMN% corresponds to chronically infected knee arthroplasties, whereas acute infections (defined as less than 3 months from index surgery or from symptom onset) can lead to much higher levels (synovial leukocyte count of 27,800 cells/µL and PMN% of 89%, based on primary total knee arthroplasties with knee aspiration within 6 weeks of surgery in a study by Bedair et al [7]) [3]. Other minor criteria involved (4) purulence of the affected joint, (5) isolation of a microorganism in one culture of periprosthetic tissue or fluid, or (6) histological examination of periprosthetic tissue for the presence of neutrophils [3]. Here, the presence of four or more minor criteria indicated a PJI. These diagnostic tests became the “gold standard” of PJI diagnosis and became ingrained in clinical practice.
Over time, as diagnostic capabilities evolved, new biomarkers for PJI were identified and were introduced into diagnostic testing. Moving forward, in 2013 an expert group convened to form the first International Consensus Meeting (ICM) on PJI [1]. The group endorsed the definition of PJI from the MSIS but added a new test to the minor criteria—the leukocyte esterase (LE) test—while defining thresholds for the minor diagnostic criteria and modifying the AAOS algorithms [1]. The LE test is a rapid test which is used to identify the presence of LE, an enzyme produced by activated neutrophils in response to inflammation (Table 1).
Notwithstanding the impressive work that was put into creating diagnostic definitions of PJI, the majority of the criteria was based on expert opinion [8]. During the last decade, diagnostic tests and techniques rapidly evolved and this coincided with the need for the development of evidence-based diagnostic criteria for PJI. As such, in 2018, Parvizi et al [9] produced the first evidence-based and validated criteria [8, 9]. Their validation study was multi-institutional and aimed to “generate an evidence-based, weight-adjusted scoring system for the definition of PJI of hip and knee, validate it on an external cohort, and compare its performance against currently available definitions” [9]. As a result, higher sensitivity of 97.7% compared to the MSIS definition (79.3%) and the ICM definition (86.9%) was demonstrated by the new criteria, with specificity remaining similar at 99.5% [9]. After the validation study [9], Shohat et al [4] published the proposed 2018 ICM criteria for PJI. The new PJI definition relied on fulfilment of at least one of two major criteria, including “two positive growth of the same organism using standard culture methods” and “sinus tract with evidence of communication to the joint or visualization of the prosthesis” [4]. If either of these major criteria were fulfilled, the joint was deemed infected. The minor criteria included either elevated serum CRP or elevated D-dimer, which was a new addition to the criteria. As standard, elevated ESR remained, and further there was the option of elevated synovial white blood cell (WBC) count, LE, or positive alpha-defensin, another new addition [4]. Further minor criteria were (1) a single positive culture, (2) a positive histology, or (3) a positive intraoperative purulence [4]. This definition of PJI relied on weighted scoring of the minor criteria and criteria for intraoperative diagnosis and bases the decision on the summed final score [4]. Here, a joint was considered infected if the combined score was ≥ 6, not infected if it was < 3, and the decision was inconclusive if the score were 3–5. In such inconclusive cases, it was recommended to consider additional molecular diagnostics, eg, next-generation sequencing (NGS) [4].
This weighted-score definition set had a moderate level of evidence and was agreed upon by 68% of delegates at the ICM in 2018, but it was not endorsed by the MSIS or the European Bone and Joint Infection Society (EBJIS) [5]. In 2021, the EBJIS [5] proposed a slightly different definition of PJI again based on clinical and blood testing, synovial fluid cytological analysis, synovial fluid biomarkers, microbiology, histology, and nuclear imaging, categorizing the results of the tests into infection unlikely, infection likely or infection confirmed [5]. The authors stated that the new definition was more sensitive than previous ones, also noting the addition of the novel “infection likely” group, which, as the authors note, prompts “clinicians to think again about cases which traditionally would not be classed as infected, but which may be” [5]. Further they commented on the addition of nuclear imaging which had increased in both knowledge and availability [5].
By this time, after over 10 years of development of PJI diagnostic guidelines, there were a couple of established working definitions; ICM 2018 and EBJIS 2021 criteria, as well as the Infectious Diseases Society of America (IDSA) 2013 guideline (not described here) [10]. As such, it was only a matter of time before the different definitions would be compared. Sigmund et al [10] performed a single-center, retrospective analysis of prospectively collected data in patients indicated for revision surgery after either a total hip or total knee arthroplasty. For each patient they performed a standardized diagnostic workup for PJI and then identified the different components of the criteria from the ICM, EBJIS, and IDSA [10]. The result favored the EBJIS definition, noting that this PJI definition significantly reduced the number of uncertain diagnoses [10].
Fast-forward to 2025, Sebastian et al [11] published an editorial ahead of the 2025 ICM where the unified PJI definition endorsed by the ICM, IDSA, MSIS, EBJIS, and European Society of Clinical Microbiology and Infectious Diseases (ESCMID)/ESCMID Study Group for Implant-Associated Infections (ESGIAI) was presented prior to publication. Sebastian et al [11] commented on the fact that it appeared from the literature that “many authors were incorrectly interpreting the criteria mentioned in the validation study as the 2018 ICM PJI definition“. As such, they reiterated the differences in the preoperative diagnosis and decision that were noted in the final 2018 ICM PJI definition [11].
As Jennings points out, now in 2025 the guidelines have been once again updated at the recently convened 3rd ICM in Turkey, considering recent developments in clinical diagnoses and testing, a landscape undergoing rapid development and change.
A closer look at the standard diagnostic tests for periprosthetic joint infection
When presented with a patient who may have PJI, the guidelines aid in diagnosis, though, as Jennings notes, some diagnostic tests may be more advantageous when PJI is chronic [2].
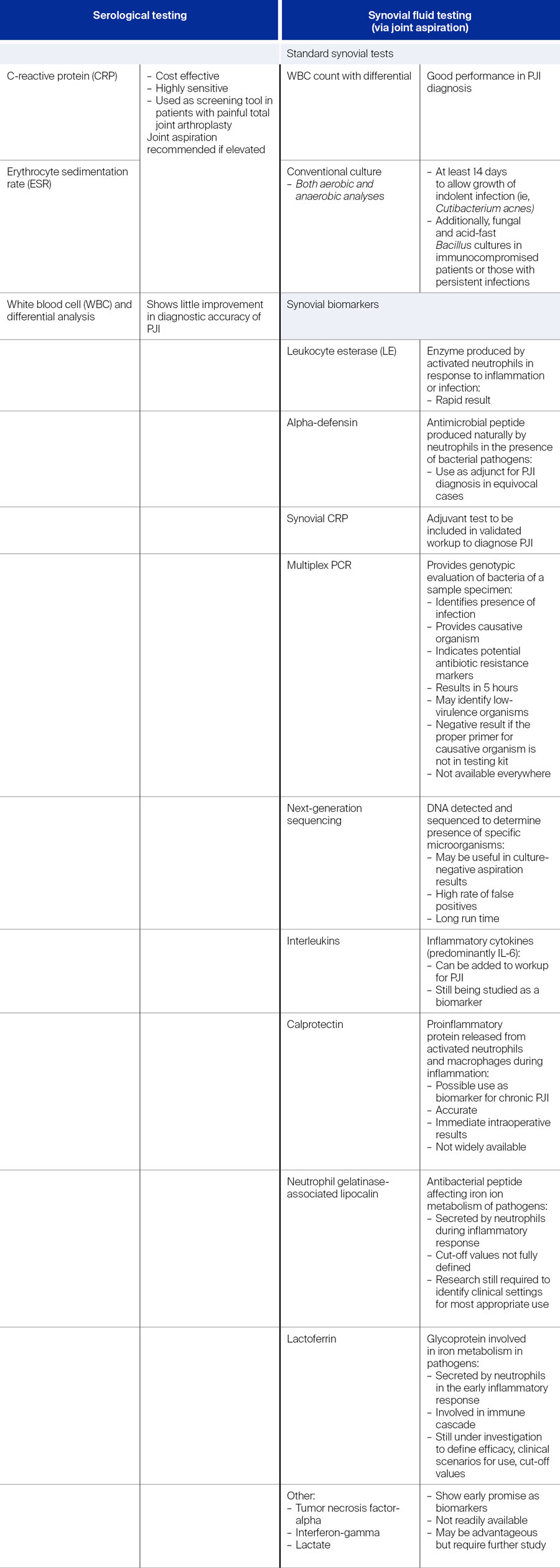
As noted above, the major criteria for PJI diagnosis according to the 2018 ICM definition [4] include at least one of the following: (1) two positive cultures of the same organism or (2) a sinus tract with evidence of communication to the joint or visualization of the prosthesis. If one of those criteria are fulfilled, the joint is infected. When it comes to the minor criteria, the testing increases slightly in complexity, with the preoperative diagnostic tests being divided into serological and synovial fluid testing [2, 4, 9] (Table 1), each with their own advantages and disadvantages. Jennings notes, “In my hands for most patients I rely heavily on synovial fluid aspiration for the diagnosis of periprosthetic infection. An elevated synovial fluid WBC has been shown in many studies to be the most sensitive for the diagnosis of infection. Obviously, our biggest struggle continues to be indolent infections, and these are the cases where some of these other tests may be advantageous to rule in or out infection.”
According to the 2018 ICM definition [4], elevated synovial WBC count or LE and positive alpha-defensin were weighted highest with 3 points each. Elevated CRP or D-dimer, a serum test, and elevated synovial PMN% were weighted with 2 points each [4]. The criteria with a 1-point score were elevated ESR, a serum test, and elevated synovial CRP [4].
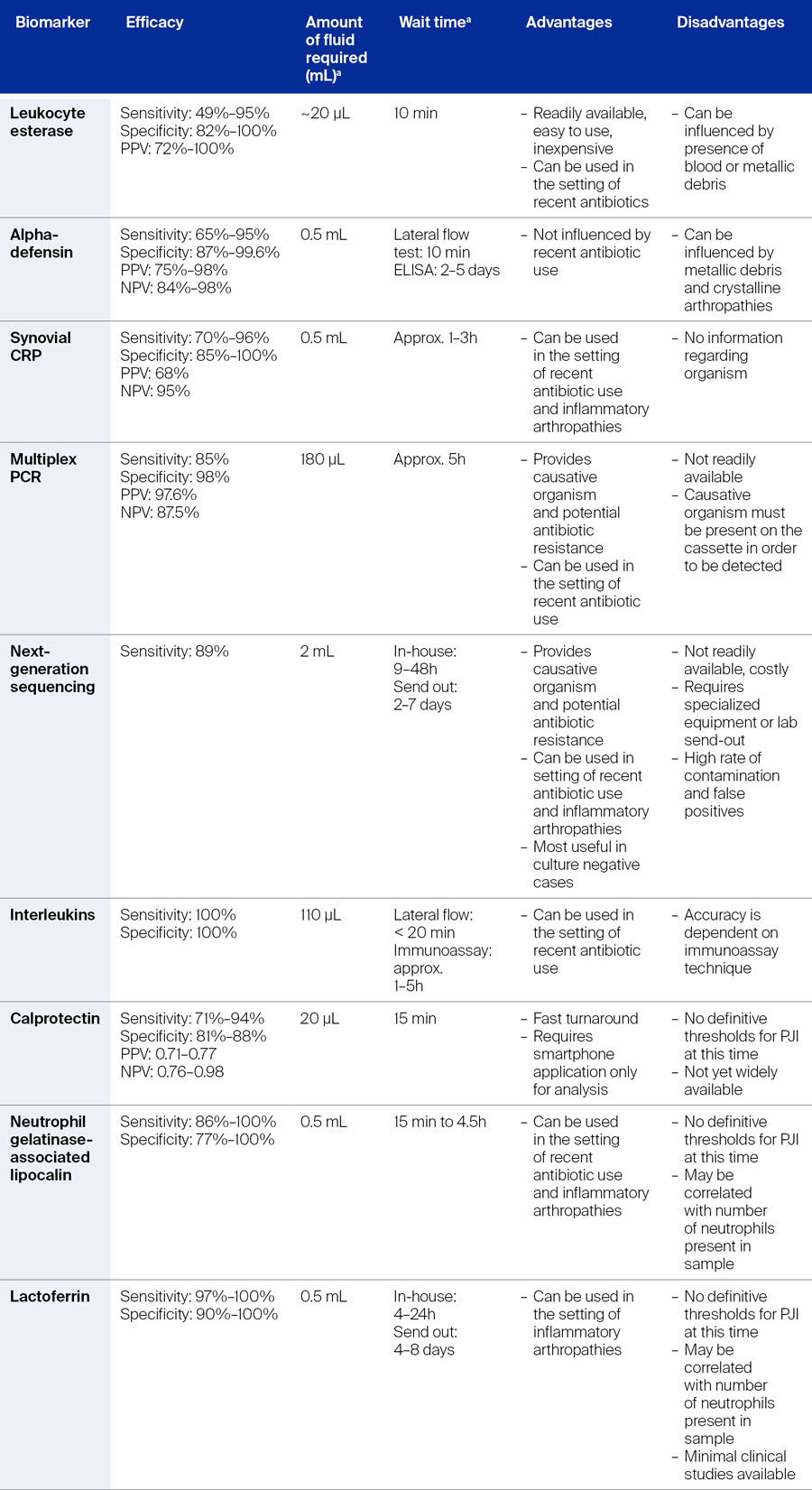
An overview of synovial biomarkers complete with their efficacy can be found in Table 2.
Serological testing
According to the 2018 ICM [4], the threshold for the minor diagnostic criteria with regard to serological testing for chronic PJI were for ESR 30 mm/h, for CRP 10 mg/L, for synovial WBC count 3,000 cells/µL and synovial PMN neutrophil 80%. At the 2025 ICM meeting which convened in May 2025 in Turkey, Pupaibool et al [12] discussed whether there should be joint-specific (total hip arthroplasty vs total knee arthroplasty) cut-offs for serological tests in diagnosing PJI. They reported that many previously conducted studies involved combined data from hip and knee PJI, were retrospective in nature, and led to complicated “joint-specific” conclusions [12]. Additionally, although the above thresholds for CRP and ESR are recommended not only by the ICM 2018 guideline but also the MSIS, some studies suggested that the thresholds were too high and led to false negatives, leading to a call for lower cut-offs [12]. For example, the ESR was removed from the 2021 EBJIS PJI definition as it was considered to offer no added value over CRP [5]. Pubaibool et al [12] reviewed available evidence to evaluate whether the joint type could influence CRP and ESR thresholds in PJI diagnosis. The authors noted that there is currently no need for separate cut-off thresholds for CRP and ESR in diagnosing hip and knee PJI, given the lack of compelling evidence to support the contrary [12]. As such, the authors recommend that the thresholds for CRP and ESR are appropriate for use in both joints (strength of recommendation: moderate) [12].
Synovial testing
The guidance provided by the ICM 2018 enabled easier diagnosis of PJI within the preoperative period [9]. However, as noted above in the comparison of definitions published by Sigmund et al [10], it was shown that the EBJIS 2021 definition had better preoperative performance than the ICM 2018 and IDSA 2013 definitions. Additionally, when using the EBJIS definition, there were fewer uncertain diagnoses allowing for easier clinical decision making. Nevertheless, the backbone of synovial testing for the preoperative period remains, and according to Quinlan and Jennings [2], joint aspiration should take place if the serological tests show any elevation or if the treating clinician suspects PJI based on the patient’s clinical picture. Note that, at the ICM 2025, Plate et al [13] concluded that there is no significant difference in synovial fluid markers in patients with PJI of the hip or knee based on current literature.
Synovial white blood cell count and leukocyte esterase
In line with the weighted-scoring definition from the ICM 2018 [4, 9], Quinlan and Jennings [2] recommend that the aspirated synovial fluid be sent for WBC count and differential, as these have shown good performance in diagnosis of PJI. Further, LE—a readily available and easy to perform test—can act as a “point-of-care” test for PJI, allowing for rapid and reliable PJI diagnosis.
Conventional culture
Aspirated synovial fluid should also be sent for conventional culture. To identify indolent infection, cultures should be allowed to grow for at least 14 days. In immunocompromised patients or those with persistent infections, cultures for fungi and acid-fast bacillus may also be requested [2].
Alpha-defensin
As a diagnostic criterium, a positive alpha-defensin test was given a score of 3 in the 2018 ICM, equating to that of elevated synovial WBC count or LE [9]. Indeed, alpha-defensin has been shown to be comparable in diagnostic accuracy to synovial WBC count and PMN% combined, but not superior [2]. During a diagnostic workup, questions may arise on the utility of synovial WBC count, LE and alpha-defensin in patients who may have inflammatory conditions, such as rheumatoid arthritis or inflammatory arthritis. It is known that such patients are at risk of PJI, with a higher incidence of PJI being found in this group. It can be challenging to diagnose PJI in these patients due to the combination of inflammation, disease stage and treatment given for their condition. This was looked at further at the recent ICM 2025 meeting, where the impact of inflammatory conditions on the accuracy of serum and synovial biomarkers for PJI diagnosis was reviewed and discussed [14]. Despite limited evidence, Linton et al [14] concluded that it appears that superior results are produced with both synovial WBC and alpha-defensin over serum biomarkers in patients with inflammatory diseases, though the authors also recognize the need for further study in larger populations [14].
Synovial polymorphonuclear neutrophils%
Synovial polymorphonuclear neutrophils% (PMN%) was considered as elevated at 70% in chronic PJI according to both the validation study and the ICM 2018 definition [4, 9]. Nevertheless, Jennings comments, “Despite this, nowadays most contemporary definitions would have PMN% at 80% or above for chronic PJI, not 70%”. In line with this, the 2021 EBJIS criteria [5, 10], which—as already noted—added a novel “infection unlikely” category to the definition criteria, defined the cut-off for PMN% levels at ≤ 65% for “infection unlikely”, > 65% or “infection likely”, and > 80% for “infection confirmed”.
Elevated synovial C-reactive protein level
As a test alone, elevated synovial C-reactive protein (CRP) may not be sufficient for the diagnosis of PJI [2]; nevertheless, as a biomarker, synovial CRP has been shown to be more accurate in diagnosing PJI than serum CRP [2]. Due to conflicting studies, it is not fully known whether synovial CRP has higher sensitivity and specificity for PJI compared to serum CRP; although some have shown this to be true, others have found similarities indicating that synovial CRP may not add diagnostic value [2]. It does, however, show promise as an adjuvant test and is recommended in the validated PJI diagnostic workup. For example, in combination, serum and synovial CRP may improve diagnostic value, particularly in chronic PJI [2], and for synovial CRP together with alpha-defensin, sensitivity and specificity are high, even in patients with systemic inflammatory disease or in settings of recent antibiotic treatment.
Exploring novel biomarkers
The above-described serum and synovial biomarkers were incorporated into the 2018 ICM. However, as described in Table 1, there are a number of novel biomarkers that have shown promise in diagnosing PJI [2]. The incorporation of these into a standard diagnostic workup may be limited not only by the availability of some of these tests, but by the cost or by sufficient clinical evidence to justify their incorporation. Jennings and Barton describe some novel biomarkers here, noting that this is not an all-inclusive list.
Calprotectin
Calprotectin became the subject of focused discussion at the ICM 2025 meeting [15]. Grzelecki et al [15], to answer the question as to whether there is a role for synovial calprotectin in the diagnosis of PJI, performed a systematic literature review including studies related to calprotectin and PJI diagnosis. Synovial calprotectin tests have shown high accuracy across different studies [15] and at the ICM 2025, it was concluded that synovial calprotectin does indeed have a role in the diagnosis of PJI, serving as a promising biomarker in the diagnosis of chronic PJI of both the hip and knee (level of evidence: strong).
Fibrinolytic markers
Novel serum biomarkers, such as fibrinogen and D-dimer (both fibrinolytic serological markers), may potentially have a role in PJI diagnosis. In infection, the level of D-dimer increases [16]. D-dimer is produced when fibrin is degraded, therefore in cases of PJI, D-dimer is produced as a result of synovial inflammation and robust fibrin production [16]. Accordingly, an increased D-dimer level can serve as a marker for increased inflammation [16]. Another fibrinolytic biomarker, plasma fibrinogen, has also emerged as biomarker, which, when compared to D-dimer, has higher specificity [16], appearing to outperform D-dimer in diagnostic precision particularly in certain scenarios. Both fibrinogen and D-dimer are involved in the same physiological process and elevations in both may occur in parallel [16]. The authors [16] noted that fibrinogen and D-dimer as diagnostic tests could be considered as valuable additions complementing the diagnostic workup of PJI. At the ICM 2025 meeting it was concluded that there is a role for both fibrinogen and D-dimer in the diagnosis of PJI, with D-dimer being recognized for sensitivity and fibrinogen for specificity (level of evidence: strong) [16].
Procalcitonin
In contrast, Abdelnasser et al [17] performed a comprehensive systematic literature review and meta-analysis to understand, based on available evidence, whether there was a role for the novel biomarker serum procalcitonin in the diagnosis of PJI. Based on the available evidence, the authors concluded that there is no role for the use of serum procalcitonin in PJI diagnosis due to its inferior performance as a marker for PJI [17].
Genetic testing for pathogen identification
In addition to the above biomarkers, novel tests using techniques to identify DNA from potential causative pathogens may be advantageous in PJI diagnosis.
Multiplex polymerase chain reaction
To detect DNA biomarkers from causative pathogens, the multiplex polymerase chain reaction (mPCR) technique can be used (Table 2). Here, genotypic evaluation of the bacteria in the sample specimen takes place. Not only can the causative pathogen be identified, but potential antibiotic resistance markers as well, even during concurrent antibiotic use. According to Quinlan and Jennings [2], the greatest diagnostic accuracy can be achieved with mPCR in conjunction with conventional cultures. One of the advantages of this technique is that multiple different causative pathogens can be tested for at the same time, and this can be done even if there is a limited volume of synovial fluid available. However, positivity is dependent on the proper primer for the causative pathogen being on the cassette; if the primer for the causative pathogen is not on the cassette used in the test, then the result is negative which may miss a diagnosis of PJI. Further, as noted by Quinlan and Jennings, availability of this test is limited [2].
Next-generation sequencing
Similar to mPCR, NGS identifies DNA in a sample. In this test all DNA in a sample specimen is detected and sequenced, allowing for determination of the presence of specific microorganisms [2]. Although NGS is still in investigation, being able to identify multiple causative pathogens and antibiotic resistance markers are an advantage [2]. Indeed, a recent study [18] found a shorter time to reporting for NGS compared with standard culture, and that NGS was more advantageous in detecting pathogens in PJI after TKA or THA. Furthermore, it could be beneficial when multiple pathogens are involved [18] and in cases where traditional culture is negative, causative pathogens may still be identified [2, 18].
Diagnosing periprosthetic joint infection intraoperatively
The scoring-based definition for PJI reported in the 2018 ICM [4, 9] could be applied in cases where the preoperative score or dry synovial aspirate is inconclusive, such as when the preoperative score was between 2 and 5 (in the validation study) [9] or 3–5 (in the 2018 ICM PJI definition [4]; for an overview see Sebastian et al [11]). In such cases the operative criteria could be used to fulfill the definition for PJI, which were categorized in descending order of importance as positive histology, purulence, and single positive culture. For increased diagnostic capability, preoperative scores were considered in the intraoperative diagnostic score leading to the use of an aggregate score of ≥ 6 equating to infected, and 4–5 as inconclusive [9]. But the question remains of what to do—particularly in the intraoperative setting—if the diagnosis is still unclear? We asked Jennings and Barton how they tackle such cases, “The importance of obtaining a definitive diagnosis of infection before surgical intervention is imperative. While intraoperative cultures may be helpful postoperatively, they are not readily available at the time of surgery. In these indolent type cases histopathology may be helpful in hospitals where there are pathologists who specialize in this area to assist with the diagnosis. For me, a quick and easy test is the LE strip test along with a repeat aspiration and stat synovial cell count and differential. It should be noted that the LE strip test must have a sample that does not contain blood or there will be a false positive test. If the aspirate is bloody, using a centrifuge has been described and is helpful in order to maintain the accuracy of this test.”
Jennings pointed out that at the 2025 ICM, although several different topics were discussed, one of the questions raised was exactly regarding doubtful cases of PJI, namely, what is the best intraoperative test to confirm or refute the diagnosis [19]. Indeed, Mortazavi et al [19] performed a comprehensive systematic literature review and identified and examined known tests that could be performed during surgery including: alpha-defensin, histopathology and frozen section, calprotectin, LE, synovial fluid analysis for WBC and PMN evaluation, and polymerase chain reaction (PCR). Based on the analysis of the available data and the diagnostic accuracy, cost efficiency, and time to result of each test, they finally recommended that the best intraoperative tests were LE, synovial fluid analysis and frozen section (strength of recommendation: moderate) [19].
Looking to the future of periprosthetic joint infection diagnosis
The development of PJI diagnostic guidelines has accompanied most practicing clinicians for many years. For those starting out their careers, they will be accompanied by the most up-to-date unified PJI definition endorsed by the ICM, IDSA, MSIS, EBJIS, and ESCMID/ESGIAI which was presented prior to publication at the 2025 ICM (forthcoming) and have the advantage of using modern tests for novel biomarkers. With the rapid technological advancements being witnessed across all fields, and further research into novel biomarkers, the future shows promise. Given that infection is the most common reason for revision hip arthroplasty (27.4%) and revision knee arthroplasty (33.1%) in the United States [20], diagnosing PJI will remain a core component of the orthopedic surgeons’ daily practice.
Conclusion
In many cases PJI continues to be a diagnostic dilemma. Indolent infection is especially challenging for both the clinician and patients. All of the aforementioned tests provide valuable information to help guide clinicians in their evaluation of patients with possible chronic PJI, but the accuracy of the tests is variable and no single test alone can be relied upon for definitive clinical decisions. Tests should be utilized in combination to accurately diagnose PJI. Lastly, the 2025 ICM has provided valuable information to the orthopedic community with the most up-to-date evidence on evaluation of PJI.
AO Recon resources
Contributing experts

Armita Armina Abedi
Department of Orthopaedic Surgery and Traumatology, Copenhagen University Hospital, Bispebjerg
Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Denmark

Kristen Barton
Midwest Orthopaedics at Rush, Rush University Medical Centre, Chicago
Department of Physical Therapy, Western University, London, Canada

Mustafa Citak
Helios ENDO-Klinik Hamburg, Hamburg, Germany

Jason Jennings
Colorado Joint Replacement, AdventHealth, Denver, Colorado
Department of Mechanical and Materials Engineering, University of Denver, Denver, Colorado
Department of Physical Therapy, University of Delaware, Newark, Delaware
This article was written by Lyndsey Kostadinov, AO Innovation Translation Center, Clinical Science, Switzerland.
References
-
Parvizi J, Gehrke T. Definition of periprosthetic joint infection. J Arthroplasty. 2014;29(7):1331.
-
Quinlan ND, Jennings JM. Joint aspiration for diagnosis of chronic periprosthetic joint infection: when, how, and what tests? Arthroplasty. 2023;5(1):43.
-
Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469(11):2992–2994.
-
Shohat N, Bauer T, Buttaro M, Budhiparama N, Cashman J, Della Valle CJ, et al. Hip and Knee Section, What is the Definition of a Periprosthetic Joint Infection (PJI) of the Knee and the Hip? Can the Same Criteria be Used for Both Joints?: Proceedings of International Consensus on Orthopedic Infections. J Arthroplasty. 2019;34(2s):S325–s327.
-
McNally M, Sousa R, Wouthuyzen-Bakker M, Chen AF, Soriano A, Vogely HC, et al. The EBJIS definition of periprosthetic joint infection. Bone Joint J. 2021;103-b(1):18–25.
-
Della Valle C, Parvizi J, Bauer TW, Dicesare PE, Evans RP, Segreti J, et al. Diagnosis of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. 2010;18(12):760–770.
-
Bedair H, Ting N, Jacovides C, Saxena A, Moric M, Parvizi J, et al. The Mark Coventry Award: diagnosis of early postoperative TKA infection using synovial fluid analysis. Clin Orthop Relat Res. 2011;469(1):34–40.
-
Mont MA, Backstein DJ, Krebs VE, Mason JB, Taunton MJ, Callaghan JJ. Evidence-Based Validation of Diagnostic Criteria for Periprosthetic Joint Infection: A Major Step Forward! J Arthroplasty. 2018;33(5):1307–1308.
-
Parvizi J, Tan TL, Goswami K, Higuera C, Della Valle C, Chen AF, et al. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J Arthroplasty. 2018;33(5):1309–1314.e1302.
-
Sigmund IK, Luger M, Windhager R, McNally MA. Diagnosing periprosthetic joint infections : a comparison of infection definitions: EBJIS 2021, ICM 2018, and IDSA 2013. Bone Joint Res. 2022;11(9):608–618.
-
Sebastian S, Parvizi J, Hofstaetter JG. Diagnostic Guidelines for Periprosthetic Joint Infection: Know the Differences. J Arthroplasty. 2025.
-
Pupaibool J, Plate JF, Hoffman A, Sebastian S, Birinci M, Kuiper J, et al. Is there a role for joint specific (hip vs knee) cut-offs for serological tests in diagnosis of prosthetic joint infection (PJI)? 3rd Meeting of the International Consensus Meeting Istanbul Turkiye 2025.
-
Plate JF, Hoffman A, Lin RT, Sebastian S, Klaber I, Grzelecki D, et al. Is there a role for joint specific (hip vs knee) cut-offs for synovial tests in diagnosis of periprosthetic joint infection? . 3rd Meeting of the International Concensus Meeting; Istanbul, Turkiye2025.
-
Linton A, Behairy W, Laoruengthana A, Ponzio D, Prieto H, Zhang X, et al. Is the accuracy of serum and/or synovial biomarkers for the diagnosis of periprosthetic joint infection (PJI) affected by the presence of inflammatory conditions? 3rd Meeting of the International Consensus Meeting Istanbul Turkiye 2025.
-
Grzelecki D, Sypień P, Birlutiu R-M, Abdelnasser MK, Bue M, von Eisenhart-Rothe R, et al. Is there a role for synovial calprotectin in the diagnosis of periprosthetic joint infection (PJI)? 3rd Meeting of the International Consensus Meeting Istanbul Turkiye 2025.
-
Shahi A, Sánchez RA, Davis C, Mathis K, Martinez S, Girones LB, et al. Is there a role for fibrinolytic serological markers, such as fibrinogen and D-dimer, in the diagnosis of periprosthetic joint infection (PJI)? 3rd Meeting of the International Consensus Meeting Istanbul Turkiye 2025.
-
Abdelnasser MK, Bayam L, Ponnampalavanar S, Lumban-Gaol I, Chinoy MA, Birlutiu R-M, et al. Is there a role for serum procalcitonin for diagnosis of periprosthetic joint infection (PJI).? 3rd Meeting of the International Consensus Meeting Istanbul Turkiye 2025.
-
Hao L, Wen P, Song W, Zhang B, Wu Y, Zhang Y, et al. Direct detection and identification of periprosthetic joint infection pathogens by metagenomic next-generation sequencing. Scientific Reports. 2023;13(1):7897.
-
Mortazavi SMJ, Hosseini-Monfared P, Abdelnasser MK, Karim MMA, M. E, Dao TT, et al. In doubtful cases for periprosthetic joint infection, what is the best intraoperative test to confirm or refute the diagnosis? 3rd Meeting of the International Consensus Meeting Istanbul Turkiye 2025.
-
American Joint Replacement Registry (AJRR): 2024 Annual Report. Rosemont, IL: American Academy of Orthopaedic Surgeons (AAOS), 2024.