Prevention of periprosthetic joint infection—have we been barking up the wrong tree?
Periprosthetic joint infection (PJI) is a devastating complication of total joint arthroplasty with often poor outcomes. Many are familiar with diagnosis and treatment of PJI which is crucial as this can impact the need for future surgeries such as revision total joint arthroplasty (TJA); yet prevention of PJI is gaining importance, not only for the surgeon but also for the patient. A growing shift in clinical focus emphasizes prevention over treatment, driven by an increased awareness of risk factors, modification of risk factors, and the significant impact of PJI following TJA on patient morbidity and mortality.
In this part of the series, Mustafa Citak from the Helios ENDO-Klinik Hamburg, Hamburg, Germany, and Armita Armina Abedi from the Department of Orthopaedic Surgery and Traumatology, Copenhagen University Hospital, Bispebjerg; Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Denmark lead us through the recently published ten steps to infection prevention, bring updates from the recent 3rd International Consensus Meeting (ICM), and provide their insights into prevention of PJI in the real world.

Mustafa Citak
Helios ENDO-Klinik Hamburg, Hamburg, Germany

Armita Armina Abedi
Department of Orthopaedic Surgery and Traumatology, Copenhagen University Hospital, Bispebjerg; Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Denmark
Periprosthetic joint infection has a wide-reaching impact
Periprosthetic joint infection is considered one of the most frequent and serious complications after TJA. According to the American Joint Replacement Registry [1], infection remained the most common indication for both revision hip arthroplasty and revision knee arthroplasty in 2024. The incidence of PJI after TJA is around 1–2%, and the number of TJAs taking place is increasing with the aging population [2]. On this topic, Citak comments, “We are seeing an increase in the number of PJIs as the number of TJAs taking place are increasing worldwide. However, the rate of PJI is still the same. This may reflect the impact of PJI prevention measures. There is a growing awareness within the surgical community regarding modifiable risk factors, such as body mass index and diabetes, and we can see that there is a definite focus on optimizing the risk factors to reduce the risk of PJI.”
According to Gehrke et al [3] PJI may lead to “surgical failure, revision surgery, amputation, or death”. This underscores the ever growing need to focus on the prevention of PJI. To this end, this article presents up-to-date knowledge on PJI prevention in TJA.
Polymicrobial infections and antibiotic resistance complicate the status quo
In patients undergoing primary and revision TJA, infections are most often detected as monomicrobial, ie, as caused by a single pathogen; however, polymicrobial infections involving two or more causative pathogens may also occur and have been associated with poorer clinical outcomes compared with infections caused by just one microbe [3]. Other issues include antimicrobial resistance and virulence, which may affect both treatment and identification of the causative pathogen [3].
In light of the complexity of both the diagnosis of PJI—discussed in Part 2 of this series— and its treatment, prevention has emerged as a central focus in TJA, not only for patients but also the surgeons [3]. It may be regarded as a change in the status quo, a shift from treatment to prevention, with infection prevention strategies now encompassing the entire surgical pathway. As Citak notes, “We are taking more care about prevention because it will not help the patient if we create an unnecessary infection, and we have the opportunity and knowledge now to consider and modify risk factors before surgery.” As Citak pointed out, “These topics are now being discussed, such as at the recent 3rd ICM in Turkey. First you identify the possible risk factors and then later you have to consider how to modify them. Now, prevention is a hot topic and the focus of avid discussion at every meeting.”
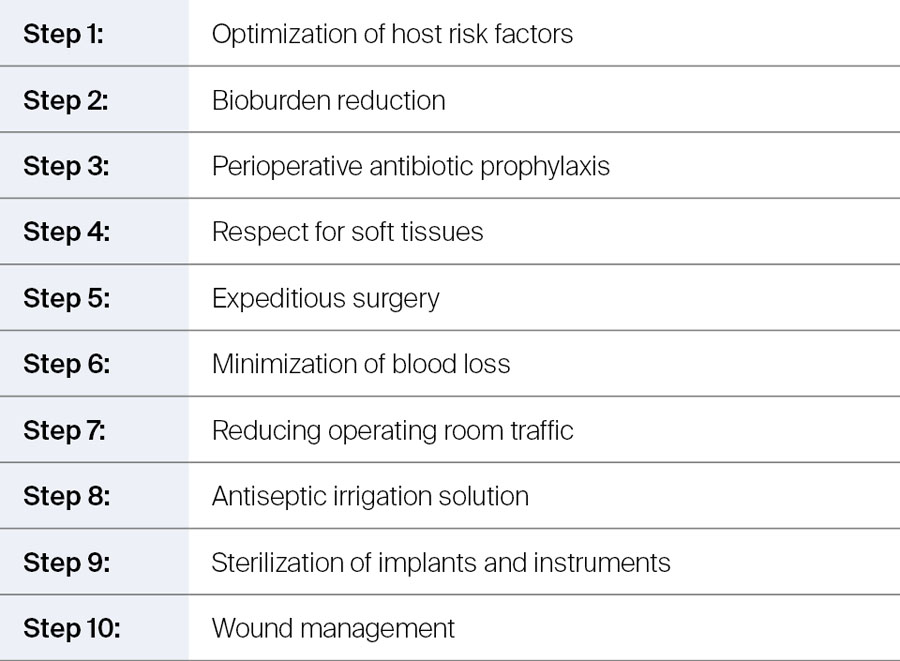
Ten steps to infection prevention
The 10-step approach to preventing surgical site infection or PJI—originally developed by Parvizi and colleagues [4, 5]—has been formally published in recent years with the aim of helping orthopedic surgeons with their infection prevention strategies. The focus is on modifiable risk factors, which can be split into two groups:
- Patient-specific risk factors (eg bioburden reduction)
- Environmental risk factors (eg, sterilization of implants and instruments).
Table 1 provides an overview of the ten steps with the following sections offering more detail.
The impact of patient risk factors
There is a wide range of host- or patient-related risk factors which may need to be considered before TJA. These range from behavioral factors such as smoking and alcohol use to metabolic conditions, chronic disease, immunological factors, nutrient deficiencies, or dermatological issues. At the recent 3rd ICM, evidence was presented showing that 80% of patients undergoing TJA have at least one modifiable risk factor for infection [6]. Indeed, research showed that there were 25 modifiable risk factors found to be associated with an increased risk of PJI [6]. As noted above, smoking, obesity, diabetes, anemia, and malnutrition were found to be the most common modifiable risk factors with other factors shown to be contributory to the risk of infection [6]. In some cases, guidance has been issued on whether TJA is appropriate in some patients—for example, undiagnosed hyperglycemia can be an issue, with TJA being advised against in those patients with elevated HbA1c (> 7%; indicating hyperglycemia, or poor glycemic control in a diabetic patient) [5]. Further, emerging research has shown that some of these patient-related risk factors such as obesity, inflammatory bowel disease, diabetes, as well as Clostridium difficile infection are linked to gut dysbiosis—discussed in Part 3 of this series—which makes patients more prone to infection. There are strategies which can be considered before TJA, such as smoking cessation, weight loss, optimization of vitamin D levels, management of immunosuppression, of malnutrition, or of anemia, which attempt to modify some of these patient risk factors.
Skin decontamination reduces microbial load
As any orthopedic surgeon knows, it has become standard to preoperatively prepare the skin to reduce the bacterial load before surgery [4, 5]. For preparation before TJA, effective strategies are in place which include using 2.0–2.5% chlorhexidine in alcohol or povidone-iodine on the surgical site as preoperative preparation [5]. Further, in the days prior to surgery the use of antiseptic soap is also recommended [4]. Other preoperative preparations such as hair removal at the surgical site are common; however, care should be taken because—as with vigorous application of antiseptics—hair removal can potentially increase the occurrence of infection through damage to the skin surface [4, 5].
Also to be considered is nasal colonization by Staphylococcus aureus. Although the available evidence is limited, research indicates that eradication of S aureus may reduce infection rates; however, the optimal method for achieving this remains a matter of ongoing discussion. The value of a standardized questionnaire to preoperatively screen for and assess the risk of methicillin-resistant S aureus (MRSA) infection has been evaluated [7], showing that it may be valuable in identifying high-risk patients. To decolonize the nose, 2% mupirocin ointment has been advised, yet at the 3rd ICM it was discussed that although mupirocin remains the recommended standard, there are concerns over resistance, cost, and efficacy. Thus, povidone-iodine could be used as a cost-effective alternative with comparable outcomes in reducing both colonization and surgical-site infection rates (strength of recommendation: moderate) [8].
Bioburden reduction (step two) is closely tied to step eight, which is the use of an antiseptic solution for irrigation during surgery to ensure effective debridement of tissues [4]. Recommendations include the use of povidone-iodine solution to irrigate the deep and subcutaneous tissues [4], with a 0.5% povidone-iodine solution commonly used for this purpose [4]. For removal of biofilms on orthopedic surfaces, a recent study [9] showed that 10% povidone-iodine solution with or without hydrogen peroxide consistently removed methicillin-sensitive S aureus and Escherichia coli biofilms in vitro and could be considered for clinical use [9]. Not only does povidone-iodine have broad-spectrum antimicrobial properties, it is effective in both chemical and mechanical decontamination, whilst remaining nontoxic to human tissues, and in particular, fibroblasts [4].
Although povidone-iodine is well known for its antimicrobial and cleansing properties, it is not used during revision procedures at either Citak’s or Abedi’s departments. According to Citak, at Helios ENDO-Klinik where he practices, saline (NaCl) is used for standard irrigation and polyhexanide solution for septic cases. Nonetheless, its potential role in future protocols warrants further investigation.
Antibiotic prophylaxis is a well-established method to prevent infection
Perioperative antibiotic prophylaxis is a standard and proven way to prevent infection during major orthopedic surgery. Commonly, first- or second-generation cephalosporins are used as a primary method of prophylaxis [4], and it is recommended that a single weight-adjusted dose of antibiotic prophylaxis should be given 30–60 minutes before incision. Citak comments, “In our clinic every patient receives a weight-adjusted single shot of antibiotics intravenously before surgery. If a patient weighs more than 120 kg, then it is recommended to use 3 g rather than 2 g cephalosporin.” Indeed, at the 3rd ICM, it was recommended that administration of all prophylactic antibiotics need to be weight based (level of evidence: strong) [10]. In addition, alternative routes of antibiotic administration are under investigation, including intrawound vancomycin powder, diluted povidone-iodine lavage, and intraarticular administration of vancomycin and tobramycin after TJA [3]; however, further studies are needed before their role in clinical practice can be defined.
Regarding postoperative antibiotic prophylaxis, while there is clear consensus on the perioperative use of antibiotics to prevent PJI, there is no consistency in the literature for continuing antibiotic prophylaxis after total hip arthroplasty (THA) [11].
Being mindful of best practices in the operating room
As outlined in the ten steps for PJI prevention (Table 1), what happens in the operating room is an important component of infection prevention. Regarding soft tissues (see Table 1, Step 4), here the focus is on using instruments in a proper fashion and not handling tissues by hand which may lead to contamination through potentially contaminated gloves. Moreover, the operating surgeon should be aware of skin tension, incision size, and minimizing the use of nonabsorbable sutures or electrocautery which have been associated with an increased risk in infection [4]. Further, it has been shown that for every 60 minutes of surgery the likelihood of surgical site infection increases by 37% [4].
Did you know?
For every 60 minutes of surgery the likelihood of surgical site infection increases by 37% [4].
This could be due to contamination of the site which may increase with time, but also due to the half-lives of the perioperatively administered antibiotics, thus additional doses may be required in prolonged operations. As such, good preoperative planning is key, particularly in complex cases which may require lengthy operating times [4, 5]. Equally important is aiming to minimize blood loss during surgery as allogeneic blood transfusions may have detrimental effects on outcomes of TJA [5] and may increase the risk of PJI [4]. Some methods include efforts to minimize operating times, where clinically appropriate, to reduce the need for an allogeneic transfusion, alongside diverse strategies to prevent unnecessary blood loss [4].
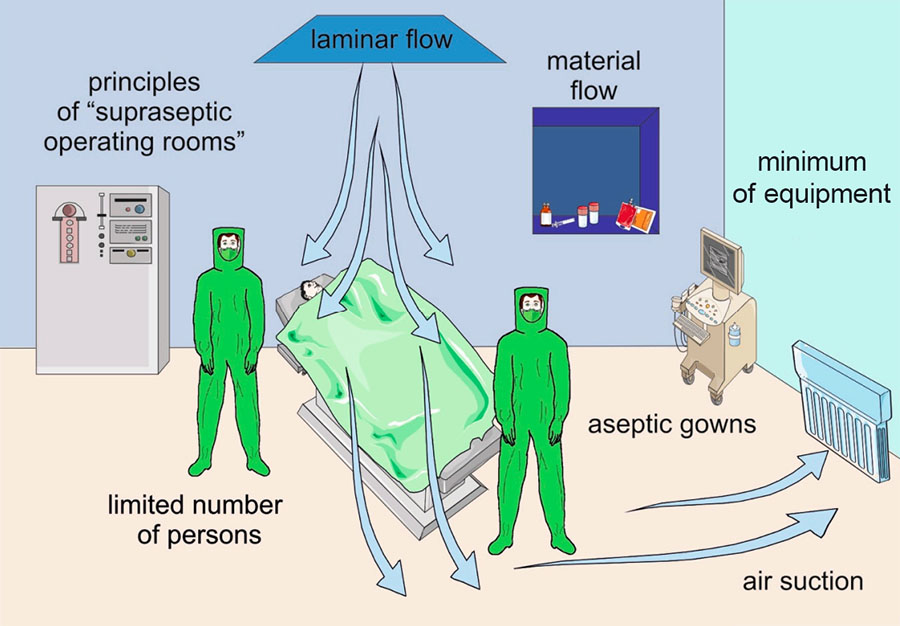
It is also known that the surgical team in the operating room, as well as the patient’s skin, are a source of bacteria which may lead to surgical-site contamination [4, 12]. For infection control, one method is to minimize the flow of staff in the operating room (Figure 1). Current guidelines on infection control recommend keeping the number of surgical personnel to a minimum without compromising patient care, but staff should also be mindful of excessive door opening or closing which may generate air currents and may cause elevated airborne bacterial counts (Figure 1) [4, 5].
Sterilization of instruments and implants is a key measure in preventing periprosthetic joint infection
One key step to preventing PJI is to ensure that implants and surgical instruments are sterile, for which there are many different methods in use [4, 5]. Recent infection prevention protocols recommend different strategies for infection prevention particularly to reduce the risk of intraoperative implant contamination, which is common. This includes minimizing exposure of the implant to the air in the operating room, glove changes before handling implants, making sure that implants do not touch the patient’s skin before implanting, and assessing the sterility of the wraps used on the surgical tray, among others [4]. This latter aspect is important as it is known that this is an area where sterility is often compromised [5].
Wound management is an important last step in preventing periprosthetic joint infection
The importance of wound closure and incision management is gaining momentum as a final measure in preventing PJI. When it comes to wound closure and management, orthopedic surgeons need to consider not only how the wound is sutured but should also aim to minimize superficial drainage, which may increase the risk of infection. Also, modern dressings, such as silver-impregnated ones or hydrofiber absorbent dressings may help reduce the rate of infection, promote wound healing, or minimize the need for medication [4, 5]. Additionally, reducing suture tension is another method for optimal wound care, where sutures following THA are recommended to be placed with the hip in extension, and after total knee arthroplasty (TKA) with the knee in 10° of flexion [4].
Interestingly, the topic of wound care was the focus of a recent Delphi consensus on both THA [13] and TKA [14]. Pertaining directly to surgical-site infection, consensus was obtained that triclosan-coated sutures may reduce the risk of infection versus conventional sutures in THA and TKA [13, 14], and sutures may lower the risk of superficial surgical-site infections compared to staples [13]. For TKA specifically, consensus was also reached on the use of silver-impregnated dressings over standard dressings, as mentioned above [14]. In this regard, these dressings may lead to fewer wound complications and decreased infections, and this is something that could be considered when performing a TKA.
Novel methods for prevention of periprosthetic joint infection
We asked Citak and Abedi about the use of modern orthopedic materials and coatings and whether they have been translated into clinical practice, “We wouldn’t say routinely; in our clinics we don’t use specific coated implants in primary or revision surgery, and even silver-coated implants are not widespread in nononcological patients. We believe these are still part of the research and haven’t made it fully into clinical practice yet.” Indeed, these novel technologies are still under investigation. Bioactive materials which could be used in TJA include polymethylmethacrylate bone cement which also may be enhanced with nanosilver or bioactive glass leading to prolonged antimicrobial effects [3]. Additionally, there are antimicrobial-coated implants such as those coated with silver nanoparticles or nitric oxide [3]. Although this technology is still under investigation, it will be interesting to see how innovative bioactive orthopedic materials or antimicrobial-coated implants may impact PJI outcomes in the future after translation into everyday clinical practice.
The importance of the gut microbiome in periprosthetic joint infection
An emerging area of interest is the patient’s microbiome, which may influence wound healing and the risk of surgical-site infections. The patient’s microbiome is theorized to indirectly affect tissue healing and the incidence of surgical-site infections [15]. While evidence mainly stems from gastrointestinal and hepatobiliary surgery, where probiotics and synbiotics have been associated with reduced postoperative infections, their role in arthroplasty remains unexplored. As such, it could be argued that maintaining gut microbiome diversity through taking probiotics before TJA may help to lower the risk of PJI through improving immune defenses, though the extent of these relationships is still being researched [15]. For a deep dive into the microbiome and PJI take a closer look at Part 3 of this series of articles.
Conclusion
As we can see, there are many steps that need to be considered when it comes to prevention of PJI. The “ten steps” framework highlights how strategies span from preoperative optimization of host-related factors, such as obesity and diabetes to intraoperative measures and postoperative attention. While recent focus has shifted toward modifying patient-related risk factors, it is important not to overlook the established value of meticulous surgical technique, perioperative prophylaxis, and infection control practices. In conclusion, novel technologies and adjunctive strategies may further enhance prevention, but they should complement rather than replace proven measures. Have we been barking up the wrong tree? Perhaps not—but this question underscores that prevention must remain our central focus. As the old adage reminds us “prevention is better than cure”, and greater efforts are needed to optimize prevention strategies for our patients.
AO Recon resources
Contributing experts

Armita Armina Abedi
Department of Orthopaedic Surgery and Traumatology, Copenhagen University Hospital, Bispebjerg
Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Denmark

Kristen Barton
Midwest Orthopaedics at Rush, Rush University Medical Centre, Chicago
Department of Physical Therapy, Western University, London, Canada

Mustafa Citak
Helios ENDO-Klinik Hamburg, Hamburg, Germany

Jason Jennings
Colorado Joint Replacement, AdventHealth, Denver, Colorado
Department of Mechanical and Materials Engineering, University of Denver, Denver, Colorado
Department of Physical Therapy, University of Delaware, Newark, Delaware
This article was written by Lyndsey Kostadinov, AO Innovation Translation Center, Clinical Science, Switzerland.
References
-
American Joint Replacement Registry (AJRR): 2024 Annual Report. Rosemont, IL: American Academy of Orthopaedic Surgeons (AAOS), 2024.
-
Ayoade F, Li D, Mabrouk A, al. e. Periprosthetic Joint Infection. [Updated 2023 Oct 14]. StatPearls [Internet]. Treasure Island [FL]: StatPearls Publishing; 2025.
-
Gehrke T, Citak M, Parvizi J, Budhiparama NC, Akkaya M. Periprosthetic joint infections: state-of-the-art. Archives of Orthopaedic and Trauma Surgery. 2024;145(1):58
-
Tarabichi S, Parvizi J. Prevention of surgical site infection: a ten-step approach. Arthroplasty. 2023;5(1):21.
-
Yilmaz MK, Celik N, Tarabichi S, Abbaszadeh A, Parvizi J. Evidence-based Approach for Prevention of Surgical Site Infection. Hip Pelvis. 2024;36(3):161–167.
-
Ahmed SO, Veloso M, Sabater-Martos M, Ploegmakers J, Jennings JM, Kaplan N, et al. What are the modifiable risk factors for postoperative infection in patients undergoing major orthopaedic surgery? 3rd Meeting of the International Consensus Meeting Istanbul Turkiye 2025.
-
Von Rehlingen-Prinz F, Röhrs M, Sandiford N, Garcia EG, Schulmeyer J, Salber J, et al. Preoperative MRSA screening using a simple questionnaire prior elective total joint replacement. Arch Orthop Trauma Surg. 2024;144(12):5157–5164.
-
Lizcano JD, Pelt C, Norambuena GA, Martinez D, Sieidelman J, Riaz T, et al. What is the optimal nasal decolonization agent for patients undergoing major orthopaedic surgery? 3rd Meeting of the International Consensus Meeting Istanbul Turkiye 2025.
-
Chao CA, Khilnani TK, Jo S, Shenoy A, Bostrom MPG, Carli AV. Not All Antiseptic Solutions Are Equivalent in Removing Biofilm: A Comparison Across Different Orthopaedic Surfaces. J Bone Joint Surg Am. 2025;107(2):127–133.
-
Shahi A, Long W, Guild G, Mathis K, Uchiyama K, Uckay I, et al. Should the administration of prophylactic antibiotics be weight-based? 3rd Meeting of the International Consensus Meeting Istanbul Turkiye 2025.
-
Kubsad S, Collins AP, Dasari SP, Chansky HA, Fernando ND, Hernandez NM. Impact of Extended Prophylactic Antibiotics on Risk of Prosthetic Joint Infection in Primary Total Hip Arthroplasty: A Matched Cohort Analysis. J Am Acad Orthop Surg. 2025;33(6):307-312.
-
Gallo J, Nieslanikova E. Prevention of Prosthetic Joint Infection: From Traditional Approaches towards Quality Improvement and Data Mining. J Clin Med. 2020;9(7).
-
Ainslie-Garcia M, Anderson LA, Bloch BV, Board TN, Chen AF, Craigie S, et al. International Delphi Study on Wound Closure and Incision Management in Joint Arthroplasty Part 2: Total Hip Arthroplasty. J Arthroplasty. 2024;39(6):1524–1529.
-
Ainslie-Garcia M, Anderson LA, Bloch BV, Board TN, Chen AF, Craigie S, et al. International Delphi Study on Wound Closure and Dressing Management in Joint Arthroplasty: Part 1: Total Knee Arthroplasty. J Arthroplasty. 2024;39(4):878–883.
-
Abedi AO, Abedi AA, Parvizi J. Prevention of surgical site infection: need to focus on the enemy within. Journal of Joint Surgery and Research. 2025;3(1):73–77.