Patellar fractures after total knee arthroplasty
Etiology
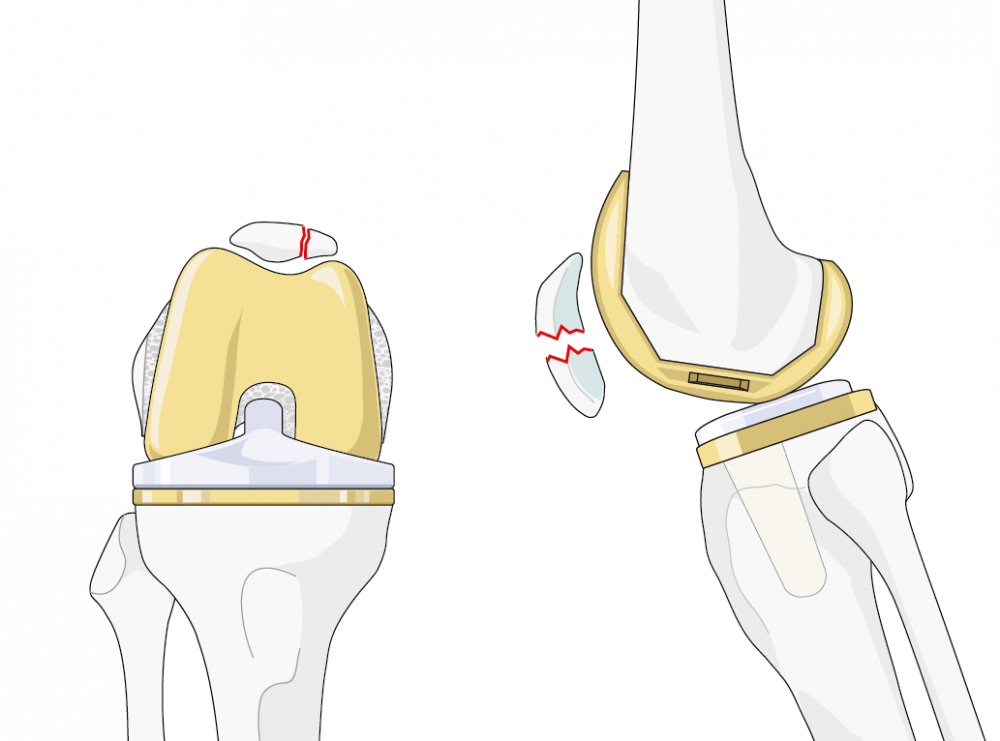
Patellar fractures (Figure 1) are one of the most common periprosthetic fractures [1]. Their incidence after primary TKA is overall approximately 1% [1, 2], but may be as high as 20% in certain patellar implants [3]. After revision surgery, incidences are roughly twice as high as after primary surgery [4].
Their etiology is multifactorial. In the minority of cases, the fractures are preceded by a traumatic event [2, 5, 6]. Often, they are asymptomatic [6, 7]. Usually, they emerge within 2 or 3 years after surgery [6, 8]. Broadly speaking, the risk factors can be categorized into patient-related, implant related, and technical.
Patient-related factors are primarily linked to bone quality, bone quantity, and loading of the implant. Besides the obvious osteoporosis and osteopenia linked to aging, they can also be induced by rheumatoid arthritis, which is thus an important risk factor [2]. This is particularly important in the presence of a very thin and eroded patella [9]. Moreover, prosthetic wear and loosening can lead to progressive osteolysis [8].
Did you know?
Most orthopedic and trauma surgeons are painfully aware of the poor bone quality they often find when operating on patients with rheumatoid arthritis. But why is this so? Clemens Gwinner, head of knee endoprosthetics at the university hospital Charité in Berlin, Germany, explains: “Rheumatoid arthritis is a systemic disease which causes joint inflammation. The inflammatory cytokine release is, amongst other effects, responsible for the increase in osteoclast activity, which ultimately leads to bone degradation.”

Clemens Gwinner
Charité—University Medicine Berlin
Berlin, Germany
Loading of the implant in the presence of correctly aligned components is mainly affected by the patient's weight, activity level [9, 10], and range of motion [11]. Of note, male sex has been identified as a risk factor for periprosthetic patellar fracture by some authors [6]. However, a likely explanation for this otherwise unexplained finding is the higher body weight and higher activity level usually found in men [6, 12]. A high degree of knee flexion has also been shown to be a risk factor [9]. The biomechanical mechanism is, that during active flexion, the stresses in the patellofemoral joint increase gradually as the knee bends. At 45–60° of flexion, the pressures on the proximal part of the patella are the highest. While deep active bending occurs less often with Western lifestyles, it is essential for activities of daily living in many Asian societies.
Implant-related factors center around patellar button design. Polyethylene components with one large central peg have shown to increase the risk of patellar fractures [4, 9]. In contrast, cemented dome-shaped all-polyethylene components were associated with the lowest patellofemoral complication rates of all designs tested [13]. The high risk associated with the central peg design is because the central peg can act as a stress riser when the patella is loaded [14]. Additionally, metal-backed components, in particular when uncemented, have been shown to correlate with an increased risk of patellar fractures [9, 13].
Of note, a native patella is generally less likely to fracture than a resurfaced one [2, 5, 9].
Did you know?
Have you also overheard debates at congresses about whether to resurface or not to resurface the patella? This controversy dates back to the early 1980s. Despite the recent advances, it remains unclear if resurfacing should be performed as a standard in primary TKA. Clemens Gwinner reserves this procedure for selected cases. These include posttraumatic or postinfectious cases, patients with patellofemoral incongruency or maltracking, inflammatory arthritis, as well as advanced patellofemoral osteoarthritis.
Besides the controversy about resurfacing during primary TKA, there is also no consensus on how to deal with preexisting patellar implants in revision surgeries.
Moreover, the thickness of the remnant patellar bone is important. Considering that the goal of patellar resurfacing is to reproduce a patellar thickness that resembles the physiological situation, and, given that most patellar components have a thickness of 8–10 mm, an equal amount of bone should be resected. Clemens Gwinner advises that, generally, the minimum thickness of the remaining bone should be no less than 10–12 mm. This also applies to revision surgery, where a minimum patellar thickness of 10 mm is recommended [14].
On the other hand, abnormal contract stresses and point loading in the patellofemoral joint can be caused by malalignment, overstuffing [14], and patellar maltracking. Likewise, an increased Q-angle and patellar subluxation may result in increased peak pressures in the patellofemoral joint [15]. Furthermore, excessive preoperative varus deformity and a low patellar height have been shown to correlate with an increased patellar fracture rate [16].
Management
The management of patellar fractures after TKA depends on the type of injury. Fractures of unresurfaced patellae are usually treated as in native knees. With an intact extensor mechanism, more articular surface disruption is acceptable, because in the case of posttraumatic osteoarthritis, later resurfacing remains an option [9]. For the treatment of resurfaced patellae, location, fracture pattern, extensor mechanism integrity, prosthetic component stability, as well as the quantity and quality of the remaining bone stock have to be considered upon choosing the treatment modality [14]. These range from nonoperative methods to surgery involving open reduction and internal fixation, up to component resection with patelloplasty as well as partial or, as a salvage procedure, complete patellectomy [6]. Various authors have proposed classifications for periprosthetic patellar fractures [6, 13, 17, 18]. The one proposed by Ortiguera and Berry is widely regarded as the most useful classification because it implements a treatment algorithm based on the characteristics of the injury [6] (Figure 3). It describes three major fracture types with two subtypes. Type I fractures are characterized by a well-fixed prosthesis with an intact extensor mechanism. Type II are characterized by a well-fixed or loose prosthesis with a disrupted extensor mechanism, type IIIa by a loose prosthesis with reasonable bone stock, and type IIIb by a loose prosthesis with poor bone stock. Poor bone stock is defined as comminuted or <10 mm thick, which would make the patella unsuitable for fixation or another resurfacing procedure.
Indications for nonsurgical treatment
There is wide consensus that patellar fractures with a well-fixed implant and intact extensor mechanism, ie, type I fractures, should be treated nonoperatively. Some authors have narrowed this recommendation down to fractures with a displacement of <2 mm [19] or have further specified it by proposing nonoperative treatment explicitly for vertical and transversal fractures with <2 mm dislocation [20].
Nonoperative treatment may consist of observation, or immobilization with a brace, splint or cast. In particular when patient noncompliance is suspected, a cylinder cast may be the treatment of choice [9].
Indications for surgery and surgical techniques
Periprosthetic patellar fractures associated with disruption of the extensor mechanism (type II) or loose prosthetic components (type III) should always be treated surgically. The same applies to fractures that are displaced for >2 mm, unless they are vertical, or comminuted with a prosthetic component that is still firmly fixed at the patellar button [20].
Surgical treatment of periprosthetic patellar fractures is demanding. Treating the fracture alone will not lead to the desired outcome if the underlying causes are not addressed. These may include patellar maltracking, disruption of the extensor mechanism, or prosthetic component loosening [12].
To choose the best treatment modality along with the optimum timing of the treatment, not only the fracture pattern, fracture location, and soft-tissue damage must be considered. Equally important are patient factors such as age, bone quality and quantity, activity level, and compliance.
The available surgical treatment modalities are numerous.
Open reduction and internal fixation (ORIF) may be achieved with tension bands, cerclages, or lag screws.
Notwithstanding, ORIF may be complicated by the presence of a prosthetic patellar component. When the prosthetic component precludes adequate exposure of the fracture, cerclage wiring with retinacular repair may be the most suitable fixation method [14].
Whether ORIF should be combined with prosthetic revision depends on the stability of the patellar component. Loose components should be removed routinely. Stable components should be left in place unless removal is required to allow adequate stabilization. Subsequent resurfacing, however, should not be undertaken if the patella has previously undergone operative fracture fixation. The implant may weaken the repair and convey greater stress on the extensor mechanism, which in turn may increase the risk of nonunion or refracture [14]. Likewise, resurfacing is not indicated in patients whose remaining bone stock is inadequate. In these cases, patellar resection arthroplasty (patelloplasty) is a better option [19]. During this procedure, all small fragments of bone are removed except for the fragment adjacent to the patellar tendon. The remaining patellar bone stock is thinned to a wafer, and, if required, the retinaculum is repaired [21]. Thus, the remnant bony shell of the patella will directly articulate with the trochlea. Potential sequels of this procedure are anterior knee discomfort or crepitus [14].
Bony avulsion fractures of the quadriceps or patellar tendon, ie, fractures at the proximal or distal patellar pole, are special insofar as they are managed just like quadriceps tendon or patellar tendon disruptions, similar to those in native knees. Fracture ends are reapproximated where possible, and, if required, the repair is augmented with suitable graft [19]. Note that the topic of extensor ruptures is described in detail in part 3 of this Surgical Insights issue.
Partial patellectomy, if required, in combination with a repair of the retinaculum or extensor mechanism, can be indicated in the presence of very small fragments, severe comminution and displacement, especially for small proximal or distal fragments [14].
Total patellectomy, on the other hand, has serious consequences. Since the patella functions as a lever arm for the quadriceps, its removal will inevitably result in significant quadriceps weakness and thus should be regarded as a salvage procedure for cases where fracture fixation appears hopeless, eg, in severe comminution or after failed ORIF [9, 14].
Outcomes and conclusions
Many publications emphasize the unfavorable outcomes of surgical treatment of periprosthetic patellar fractures. However, one should bear in mind that the choice of the treatment method is usually dictated by the fracture type. Therefore, it appears more realistic that the outcomes are mainly determined by the type of fracture [6]. Based on the low incidence of periprosthetic patellar fractures, no randomized studies comparing different treatment methods have been published, so the current best evidence relies on case series. Most of these case series are small, ie, they report on less than 30 patients. Moreover, the study populations typically comprise a mix of fracture types and treatment methods which are often not reported in a stratified manner. This is a further limitation of the robustness of the available evidence.
The one universal conclusion from the available literature is that fractures with well-fixed prostheses and an intact extensor mechanism have a good prognosis when treated nonoperatively. In the largest series on patellar fractures published so far, good outcomes were reported for 93% of patients [6, 22]. Interestingly, good outcomes were also achieved in patients with fibrous rather than osseous union, in whom the patella remained functional.
Fractures requiring operative treatment are typically associated with high complication rates and poor outcomes.
Chalidis et al performed a systematic literature review and identified seven studies reporting on ORIF for periprosthetic patellar fracture fixation. The mean nonunion rate in these studies was as high as 92% and the clinical results were poor in most patients [2].
Two of these publications stand out due to the large population size. Keating et al published a retrospective study on a consecutive series of 4,583 primary TKAs and identified 177 patellar fractures in 135 patients [22], and Ortiguera and Berry published a retrospective review where they identified 85 fractures in 77 patients from a consecutive cohort of 12,464 TKAs [6].
Keating et al categorized their patients according to fracture types partially overlapping with the classification proposed by Ortiguera and Berry (Figure 3). Of 22 fractures that were vertical with a stable implant and intact extension mechanism (type 1) and 21 fractures with a disruption of the extensor mechanism of less than 1 cm (type 2a), all but two were treated conservatively with results rated as good. According to the Knee Society clinical rating system [23], knee scores were 85 and 92 points, the average pain scores were 44 and 49 points, and flexion was 120° and 117° in patients with type 1 and type 2a fractures, respectively. No extensor lag was seen in any of these patients. One patient in each group underwent excision of an extruded patellar button, and a deep infection developed in one of them.
Of the 17 fractures with separation of the fragments of >1 cm (type 2b), 14 were treated nonoperatively. In three of those cases, an extensor lag remained. Of the three patients treated operatively, two patients were treated with ORIF. Even though the treatment did not result in bony union, the clinical results were described as good. In the third patient, a loose extruded patellar button was excised, and the patient developed an infection, but no further outcome was reported.
Of the 114 fractures with an intact extensor mechanism and an unstable implant (type 3), 108 were treated nonoperatively. At the final follow-up, they had an average pain score of 43 points, an average knee score of 83 points, and an average range of knee flexion of 116°.
Six of the type 3 fractures were treated operatively, two had a subsequent deep wound infection, and one had secondary component loosening 6 months postoperatively.
The authors concluded that patients treated nonoperatively had adequate pain and function scores with minimal, if any, extensor lag and advocated that in most cases patellar fractures in TKA patients could best be treated nonoperatively [22].
Ortiguera and Berry categorized their patients according to the classification system described above (Figure 3). Of 38 patients with type I fractures, they treated 37 with observation, a brace, or a cast, and one patient with resection of the patellar component. Nonoperative treatment failed for only one knee, which required excision of an ununited marginal fracture. There was no pain, patellar instability, extensor instability or weakness at the time of the last follow-up in 31 (82%) of the 38 fractures. The latest mean pain and function scores according to the Knee Society clinical rating system [23] were 83 and 68 points, respectively.
There were 12 type II fractures, ie, fractures with a disrupted extensor mechanism. One was treated nonoperatively with prolonged immobilization. At the 5-year follow-up, the patient had no pain and a 5° extensor lag. The other eleven fractures were treated operatively. Six were treated with ORIF, but only one resulted in fracture union. The five remaining type II fractures were treated with partial patellectomy and tendon advancement to bone. Of these, three had complications that required further surgery. Overall, there were complications in six of the 12 type II fractures. Five needed further surgery, and seven had pain, patellar instability, extensor instability or weakness. The mean pain and function scores were 80 and 57 points, respectively, at the last follow-up.
Twenty-eight fractures were categorized as type III fractures, ie, with a loosened patellar component. Twelve were type IIIa, ie, with reasonable bone stock. Of these, four were treated with observation, and eight were treated operatively. Only two healed. Seven knees had complications, three needed further surgery, seven had persistent pain, patellar instability, extensor instability or weakness. All five knees treated with patellar component resection and internal fixation had complications, and two required further surgery. Of the four fractures treated nonoperatively, two were symptomatic at the time of follow-up.
Sixteen fractures were type IIIb fractures, ie, with poor bone stock. Of these, four were treated with observation, and only one of these had residual pain attributable to the patella. Twelve knees were treated operatively. Of these, seven had persistent pain, patellar instability, extensor instability or weakness, and one patient had operative complications.
Of all 28 type III fractures, eight had complications, three had a reoperation, and 15 were symptomatic at the last follow-up. The mean pain and function scores at the latest follow-up were 78 and 53 points, respectively.
The authors concluded that fractures associated with a stable implant and an intact extensor mechanism could be treated successfully nonoperatively, with minimal complications. When operative treatment was required, it was associated with a high rate of complications and reoperations.
In summary, the clinical results of treating patellar fractures in TKA patients vary greatly. Results after nonoperative treatment appear to be much better than after operative treatment. However, the treatment modality is usually determined by the underlying fracture pattern. No studies comparing different methods in the same fracture types in an unbiased sample have been published so far. Some authors recommend treating displaced fractures operatively, especially in the presence of an extensor lag [19, 24]. Other authors, in the presence of the unfavorable surgical results, argue that in displaced fractures even a substantial extensor lag may be acceptable as long as the patellar component is well fixed [25].
This controversy along with the uncertain outcomes underline the difficulties in treating periprosthetic patellar fractures. Against this background, it becomes clear that each decision has to be made on an individual basis and that paying extra attention during surgery is worth the extra effort.
Additional AO resources on this topic
Access videos, tools, and other assets to learn more about this topic.
- Video: Extensor Mechanism Problems after Total Knee Arthroplasty
- Video: Managing Deformity in Total Knee Arthroplasty
- Video: The Difficult Primary Total Knee Arthroplasty
- Video: Tips and Tricks to Improve Total Knee Arthroplasty Outcomes
- Upcoming events: AO Recon Course finder
Contributing experts
This series of articles was created with the support of the following specialists (in alphabetical order):

Guillermo Bonilla
Fundación Santa Fe de Bogotá University Hospital
Bogotá, Colombia

Clemens Gwinner
Charité—University Medicine Berlin
Berlin, Germany

Yixin Zhou
Beijing Jishuitan Hospital
Beijing, China
This issue was written by Elke Rometsch, AO Innovation Translation Center, Clinical Science, Switzerland.
References
- Mulcahy H, Chew FS. Current concepts in knee replacement: complications. AJR Am J Roentgenol. 2014 Jan;202(1):W76–86.
- Chalidis BE, Tsiridis E, Tragas AA, et al. Management of periprosthetic patellar fractures. A systematic review of literature. Injury. 2007 Jun;38(6):714–724.
- Chan JY, Giori NJ. Uncemented Metal-Backed Tantalum Patellar Components in Total Knee Arthroplasty Have a High Fracture Rate at Midterm Follow-Up. J Arthroplasty. 2017 Aug;32(8):2427–2430.
- Berry DJ. Epidemiology: hip and knee. Orthop Clin North Am. 1999 Apr;30(2):183–190.
- Assiotis A, To K, Morgan-Jones R, et al. Patellar complications following total knee arthroplasty: a review of the current literature. Eur J Orthop Surg Traumatol. 2019 Dec;29(8):1605–1615.
- Ortiguera CJ, Berry DJ. Patellar fracture after total knee arthroplasty. J Bone Joint Surg Am. 2002 Apr;84(4):532–540.
- Bourne RB. Fractures of the patella after total knee replacement. Orthop Clin North Am. 1999 Apr;30(2):287–291.
- Roth A, Ghanem M, Fakler J. [Patella fractures in knee arthroplasty]. Orthopade. 2016 May;45(5):416–424.
- Parvizi J, Kim KI, Oliashirazi A, et al. Periprosthetic patellar fractures. Clin Orthop Relat Res. 2006 May;446:161–166.
- Papalia R, Vasta S, D'Adamio S, et al. Complications involving the extensor mechanism after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2015 Dec;23(12):3501–3515.
- Insall JN, Lachiewicz PF, Burstein AH. The posterior stabilized condylar prosthesis: a modification of the total condylar design. Two to four-year clinical experience. J Bone Joint Surg Am. 1982 Dec;64(9):1317–1323.
- Tharani R, Nakasone C, Vince KG. Periprosthetic fractures after total knee arthroplasty. J Arthroplasty. 2005 Jun;20(4 Suppl 2):27–32.
- Healy WL, Wasilewski SA, Takei R, et al. Patellofemoral complications following total knee arthroplasty. Correlation with implant design and patient risk factors. J Arthroplasty. 1995 Apr;10(2):197–201.
- Sheth NP, Pedowitz DI, Lonner JH. Periprosthetic patellar fractures. J Bone Joint Surg Am. 2007 Oct;89(10):2285–2296.
- Huberti HH, Hayes WC. Patellofemoral contact pressures. The influence of q-angle and tendofemoral contact. J Bone Joint Surg Am. 1984 Jun;66(5):715–724.
- Mouton J, Gaillard R, Bankhead C, et al. Increased Patellar Fracture Rate in Total Knee Arthroplasty With Preoperative Varus Greater Than 15°: A Case-Control Study. J Arthroplasty. 2018 Dec;33(12):3685–3693.
- Goldberg VM, Figgie HE, 3rd, Inglis AE, et al. Patellar fracture type and prognosis in condylar total knee arthroplasty. Clin Orthop Relat Res. 1988 Nov(236):115–122.
- Rorabeck CH, Angliss RD, Lewis PL. Fractures of the femur, tibia, and patella after total knee arthroplasty: decision making and principles of management. Instr Course Lect. 1998;47:449–458.
- Sarmah SS, Patel S, Reading G, et al. Periprosthetic fractures around total knee arthroplasty. Ann R Coll Surg Engl. 2012 Jul;94(5):302–307.
- Windsor RE, Scuderi GR, Insall JN. Patellar fractures in total knee arthroplasty. J Arthroplasty. 1989;4 Suppl:S63–67.
- Laskin RS. Management of the patella during revision total knee replacement arthroplasty. Orthop Clin North Am. 1998 Apr;29(2):355–360.
- Keating EM, Haas G, Meding JB. Patella fracture after post total knee replacements. Clin Orthop Relat Res. 2003 Nov(416):93–97.
- Insall JN, Dorr LD, Scott RD, et al. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989 Nov(248):13–14.
- Hozack WJ, Goll SR, Lotke PA, et al. The treatment of patellar fractures after total knee arthroplasty. Clin Orthop Relat Res. 1988 Nov(236):123–127.
- Dennis DA. Periprosthetic fractures following total knee arthroplasty. Instr Course Lect. 2001;50:379–389.