Treating periprosthetic joint infection: adopting an individualized approach
Preview
How should a clinician tackle the management and treatment of a periprosthetic joint infection (PJI)? An individualized approach must be developed for each patient, one that integrates appropriate surgical intervention and antimicrobial strategies. The complexities of treating a biofilm infection calls for careful consideration of each case, addressing the acute or chronic nature of infection.
Compared to primary arthroplasty and revision for aseptic loosening, surgical revision for PJI has significantly higher associated costs and higher demands on physician resources [1]. A study of 465,209 hip revisions in the US between 2003 and 2013 found the PJI prevalence to be 15% with hospitalization costs of USD 31,529 [2]. When indirect treatment costs, such as lost wages, were taken into account, another estimate determined the base cost of treating an infected total hip arthroplasty (THA) to be between USD 389,307 and USD 474, 004, depending on the age of the patient at the time of infection [3].
See Part 1 of this article series for further elaboration of PJI risk factors and pre-, intra-, and postoperative infection prevention strategies. Part 2 looks at different components of diagnosing PJI, culminating in a recommended diagnostic algorithm.
Did you miss AO Recon’s webinar on periprosthetic joint infection (PJI)?
In June 2019, AO Recon gathered an online community of close to 200 surgeons for an interactive information session and Q&A led by Olivier Borens, Head of Septic Surgery and Head of Traumatology at the Centre Hospitalier Universitaire Vaudois (Lausanne, Switzerland) and chat moderator Andrej Trampuz, Infectious Diseases Consultant in Septic Surgery at Charité–Universitätsmedizin (Berlin, Germany) on the topic of infection after joint arthroplasty.
Treatment goals
The goal in treating a PJI is to deliver a pain-free and functional joint, which can be achieved by eliminating the infection [4]. Treatment plans should be customized to each individual patient and contingent on the microorganism(s) responsible for the infection [5]. These are simple statements to make yet the pernicious nature of device biofilm, coupled with antibiotic resistance, can make a conclusive diagnosis and successful treatment a challenge.
Treatment success rates
Compared to hospital admissions for aseptic loosening, PJI patients have a 2-times higher chance of in-hospital mortality. Treatment of PJI cases often requires multiple surgical admissions and the risk of mortality accumulates with each surgery [6]. Infection of implanted prosthetic joints is more often associated with revisions than with primary arthroplasty [7].
The rate of PJI eradication varies. Sidhu et al reported an overall rate of 50% after two years and 38.9% after five, pointing out that if there is a polymicrobial infection that includes a fungal component, patients are less likely to have their infection cured [8]. A fungal PJI has lower treatment success rates [9]. Akgun et al were able to obtain a 3 year infection-free survival rate of 89.3% in 84 hip PJI patients who underwent two-stage prosthesis exchange [10]. Similarly, Aboltins et al reported a 2 year infection-free survival of 87% in 41 hip and knee PJI patients [11].
Both abovementioned studies conducted patient follow up for 3 and 2 years, respectively. It has been suggested that follow up of any less than 10 years will not capture late PJI, leading to underreporting of its occurrence [7].
Treatment options: two elements—surgery and antibiotics
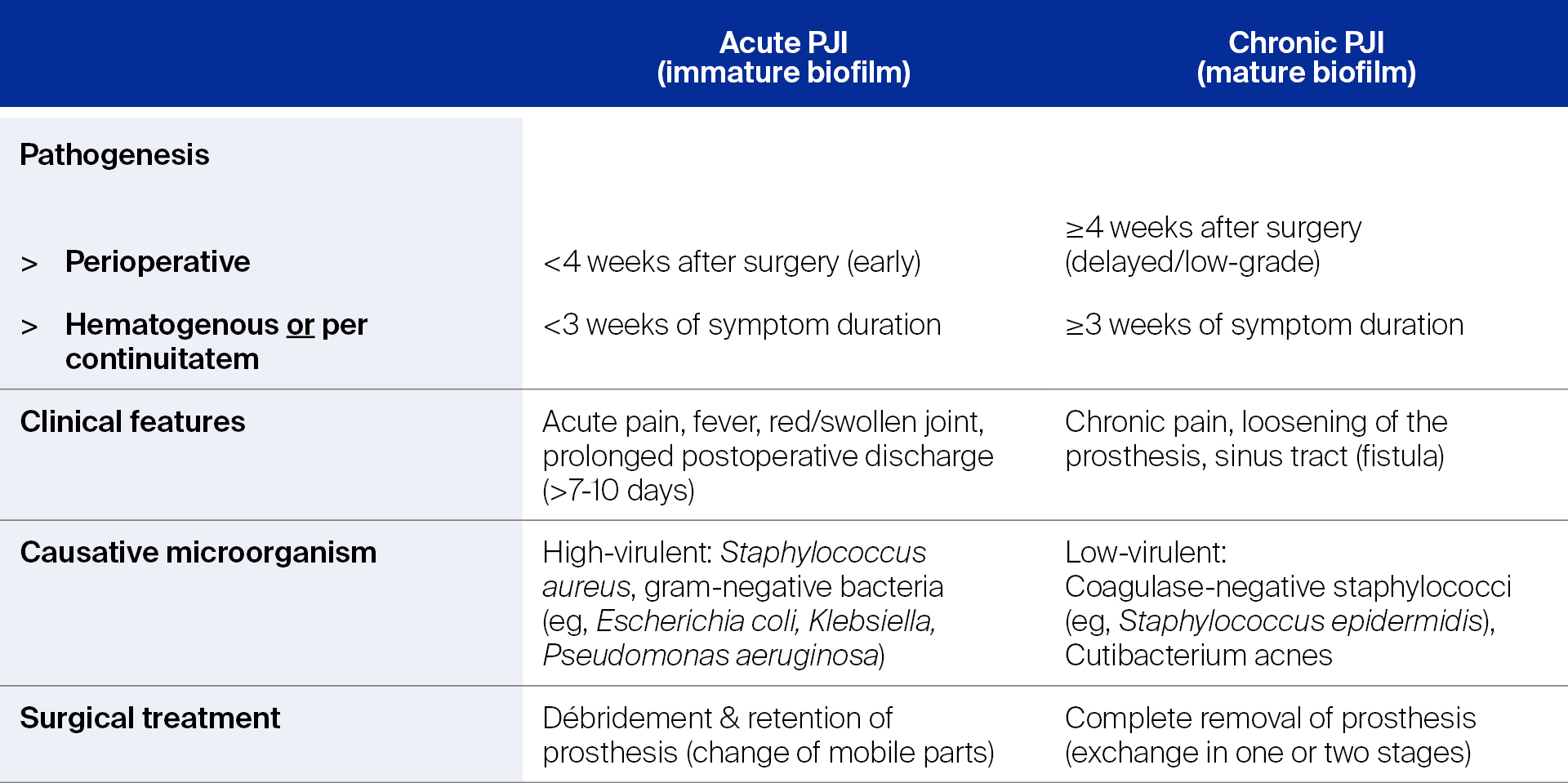
Depending on how much time has passed from implantation, or the length of symptoms, PJI is classified as either acute or chronic. This designation also indicates the maturity stage of the pathogenic biofilm, the virulence of the microorganism(s), and is associated with different clinical features [12]. See Table 1. This classification also provides direction on how to begin a treatment plan, which has two interrelated components—surgery and antibiotics. Let’s look at surgical options first.
Table 1. Classification of prosthetic joint infection (PJI) and associated surgical treatment option(s)
Read the full article with your AO login
- SURGICAL treatment strategies
- Debridement, antibiotics, and implant retention (DAIR)
- Surgical controversies: not everyone agrees
- One-stage exchange
- Two-stage exchange
- Three-stage exchange
- If it doesn’t work
- ANTIMICROBIAL treatment recommendations
- Suppressive antibiotic treatment
- Antibiotic holidays: yes or no? No
- PRO-IMPLANT Foundation recommended treatment algorithm
- New innovations—the future looks bright
- Conclusion
- References
Additional AO resources on this topic
Access videos, tools, and other assets to learn more about this topic.
- Video: Updates in Infection Management after TKA
- Video: Infection After Joint Arthroplasty
- Further reading: Fracture-related infection: new consensus on diagnosis and treatment
- Upcoming events: AO Recon Course finder
Contributing experts
This series of articles was created with the support of the following specialists (in alphabetical order):

Olivier Borens
University Hospital Lausanne
Lausanne, Switzerland

Nora Renz
Inselspital—University Hospital Bern
Bern, Switzerland

Andrej Trampuz
Charité—University Medicine Berlin
Berlin, Germany
This issue was created by Word+Vision Media Productions, Switzerland.
References
- Bozic KJ, Ries MD. The impact of infection after total hip arthroplasty on hospital and surgeon resource utilization. J Bone Joint Surg Am. 2005 Aug;87(8):1746–1751.
- Brochin RL, Phan K, Poeran J, et al. Trends in Periprosthetic Hip Infection and Associated Costs: A Population-Based Study Assessing the Impact of Hospital Factors Using National Data. J Arthroplasty. 2018 Jul;33(7S):S233–S238.
- Parisi TJ, Konopka JF, Bedair HS. What is the Long-term Economic Societal Effect of Periprosthetic Infections After THA? A Markov Analysis. Clin Orthop Relat Res. 2017 Jul;475(7):1891–1900.
- Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004 Oct 14;351(16):1645–1654.
- Li C, Renz N, Trampuz A. Management of Periprosthetic Joint Infection. Hip Pelvis. 2018 Sep;30(3):138–146.
- Shahi A, Tan TL, Chen AF, et al. In-Hospital Mortality in Patients With Periprosthetic Joint Infection. J Arthroplasty. 2017 Mar;32(3):948–952 e1.
- Kokko MA, Abdel MP, Berry DJ, et al. A retrieval analysis perspective on revision for infection. Arthroplast Today. 2019 Sep;5(3):362–370.
- Sidhu MS, Cooper G, Jenkins N, et al. Prosthetic fungal infections: poor prognosis with bacterial co-infection. Bone Joint J. 2019 May;101-B(5):582–588.
- Theil C, Schmidt-Braekling T, Gosheger G, et al. Fungal prosthetic joint infection in total hip or knee arthroplasty: a retrospective single-centre study of 26 cases. Bone Joint J. 2019 May;101-B(5):589–595.
- Akgun D, Muller M, Perka C, et al. High cure rate of periprosthetic hip joint infection with multidisciplinary team approach using standardized two-stage exchange. J Orthop Surg Res. 2019 Mar 13;14(1):78.
- Aboltins C, Dowsey M, Peel T, et al. Good quality of life outcomes after treatment of prosthetic joint infection with debridement and prosthesis retention. J Orthop Res. 2016 May;34(5):898–902.
- Izakovicova P, Borens O, Trampuz A. Periprosthetic joint infection: current concepts and outlook. EFORT Open Rev. 2019 Jul;4(7):482–494.
- Dettmers R, Brekelmans W, Leijnen M, et al. Negative Pressure Wound Therapy With Instillation and Dwell Time Used to Treat Infected Orthopedic Implants: A 4-patient Case Series. Ostomy Wound Manage. 2016 Sep;62(9):30–40.
- Wang L, Xu X, Cao JG, et al. Negative pressure wound therapy in total hip and knee arthroplasty: a meta-analysis. J Comp Eff Res. 2019 Jul;8(10):791–797.
- Newman JM, Siqueira MBP, Klika AK, et al. Use of Closed Incisional Negative Pressure Wound Therapy After Revision Total Hip and Knee Arthroplasty in Patients at High Risk for Infection: A Prospective, Randomized Clinical Trial. J Arthroplasty. 2019 Mar;34(3):554–559 e1.
- Zhang C, Yan CH, Chan PK, et al. Polyethylene Insert Exchange Is Crucial in Debridement for Acute Periprosthetic Infections following Total Knee Arthroplasty. J Knee Surg. 2017 Jan;30(1):36-41.
- Sousa R, Abreu MA. Treatment of Prosthetic Joint Infection with Debridement, Antibiotics and Irrigation with Implant Retention - a Narrative Review. J Bone Jt Infect. 2018;3(3):108–117.
- Shaw JD, Miller S, Plourde A, et al. Methylene Blue-Guided Debridement as an Intraoperative Adjunct for the Surgical Treatment of Periprosthetic Joint Infection. J Arthroplasty. 2017 Dec;32(12):3718–3723.
- Vahedi H, Aali-Rezaie A, Shahi A, et al. Irrigation, Debridement, and Implant Retention for Recurrence of Periprosthetic Joint Infection Following Two-Stage Revision Total Knee Arthroplasty: A Matched Cohort Study. J Arthroplasty. 2019 Aug;34(8):1772–1775.
- Lowik CAM, Parvizi J, Jutte PC, et al. Debridement, antibiotics and implant retention is a viable treatment option for early periprosthetic joint infection presenting more than four weeks after index arthroplasty. Clin Infect Dis. 2019 Aug 31.
- Grammatopoulos G, Bolduc ME, Atkins BL, et al. Functional outcome of debridement, antibiotics and implant retention in periprosthetic joint infection involving the hip: a case-control study. Bone Joint J. 2017 May;99-B(5):614–622.
- Abrman K, Musil D, Stehlik J. [Treatment of Acute Periprosthetic Infections with DAIR (Debridement, Antibiotics and Implant Retention) - Success Rate and Risk Factors of Failure]. Acta Chir Orthop Traumatol Cech. 2019;86(3):181–187.
- Trampuz A, Zimmerli W. Prosthetic joint infections: update in diagnosis and treatment. Swiss Med Wkly. 2005 Apr 30;135(17-18):243–251.
- Kuo FC, Goswami K, Klement MR, et al. Positive Blood Cultures Decrease the Treatment Success in Acute Hematogenous Periprosthetic Joint Infection Treated With Debridement, Antibiotics, and Implant Retention. J Arthroplasty. 2019 Jul 1.
- Faour M, Sultan AA, George J, et al. Arthroscopic irrigation and debridement is associated with favourable short-term outcomes vs. open management: an ACS-NSQIP database analysis. Knee Surg Sports Traumatol Arthrosc. 2019 Oct;27(10):3304–3310.
- Liu CW, Kuo CL, Chuang SY, et al. Results of infected total knee arthroplasty treated with arthroscopic debridement and continuous antibiotic irrigation system. Indian J Orthop. 2013 Jan;47(1):93–97.
- Chung JY, Ha CW, Park YB, et al. Arthroscopic debridement for acutely infected prosthetic knee: any role for infection control and prosthesis salvage? Arthroscopy. 2014 May;30(5):599–606.
- de Vries L, van der Weegen W, Neve WC, et al. The Effectiveness of Debridement, Antibiotics and Irrigation for Periprosthetic Joint Infections after Primary Hip and Knee Arthroplasty. A 15 Years Retrospective Study in Two Community Hospitals in the Netherlands. J Bone Jt Infect. 2016;1:20–24.
- Leta TH, Lygre SHL, Schrama JC, et al. Outcome of Revision Surgery for Infection After Total Knee Arthroplasty: Results of 3 Surgical Strategies. JBJS Rev. 2019 Jun;7(6):e4.
- Gehrke T, Zahar A, Kendoff D. One-stage exchange: it all began here. Bone Joint J. 2013 Nov;95-B(11 Suppl A):77–83.
- Pangaud C, Ollivier M, Argenson JN. Outcome of single-stage versus two-stage exchange for revision knee arthroplasty for chronic periprosthetic infection. EFORT Open Rev. 2019 Aug;4(8):495–502.
- Ilchmann T, Zimmerli W, Ochsner PE, et al. One-stage revision of infected hip arthroplasty: outcome of 39 consecutive hips. Int Orthop. 2016 May;40(5):913–918.
- George DA, Haddad FS. One-Stage Exchange Arthroplasty: A Surgical Technique Update. J Arthroplasty. 2017 Sep;32(9S):S59–S62.
- Svensson K, Rolfson O, Karrholm J, et al. Similar Risk of Re-Revision in Patients after One- or Two-Stage Surgical Revision of Infected Total Hip Arthroplasty: An Analysis of Revisions in the Swedish Hip Arthroplasty Register 1979(-)2015. J Clin Med. 2019 Apr 10;8(4).
- Beswick AD, Elvers KT, Smith AJ, et al. What is the evidence base to guide surgical treatment of infected hip prostheses? systematic review of longitudinal studies in unselected patients. BMC Med. 2012 Feb 16;10:18.
- Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013 Jan;56(1):e1–e25.
- Abdelaziz H, Gruber H, Gehrke T, et al. What are the Factors Associated with Re-revision After One-stage Revision for Periprosthetic Joint Infection of the Hip? A Case-control Study. Clin Orthop Relat Res. 2019 May 17.
- PRO-IMPLANT Foundation. Pocket Guide to Diagnosis & Treatment of Periprosthetic Joint Infection (PJI). Version 8. 2019.
- Chen AF, Parvizi J. Antibiotic-loaded bone cement and periprosthetic joint infection. J Long Term Eff Med Implants. 2014;24(2-3):89–97.
- Edelstein AI, Okroj KT, Rogers T, et al. Nephrotoxicity After the Treatment of Periprosthetic Joint Infection With Antibiotic-Loaded Cement Spacers. J Arthroplasty. 2018 Jul;33(7):2225–2229.
- Yayac M, Rondon AJ, Tan TL, et al. The Economics of Antibiotic Cement in Total Knee Arthroplasty: Added Cost with No Reduction in Infection Rates. J Arthroplasty. 2019 Sep;34(9):2096–2101.
- Vadiee I, Backstein DJ. The Effectiveness of Repeat Two-Stage Revision for the Treatment of Recalcitrant Total Knee Arthroplasty Infection. J Arthroplasty. 2019 Feb;34(2):369–374.
- Gehrke T, Alijanipour P, Parvizi J. The management of an infected total knee arthroplasty. Bone Joint J. 2015 Oct;97-B(10 Suppl A):20–29.
- Jacqueline C, Caillon J. Impact of bacterial biofilm on the treatment of prosthetic joint infections. J Antimicrob Chemother. 2014 Sep;69 Suppl 1:i37–i40.
- Donlan RM. Role of biofilms in antimicrobial resistance. ASAIO J. 2000 Nov-Dec;46(6):S47–S52.
- Escudero-Sanchez R, Senneville E, Digumber M, et al. Suppressive antibiotic therapy in prosthetic joint infections: A multicentre cohort study. Clin Microbiol Infect. 2019 Sep 17.
- Wouthuyzen-Bakker M, Nijman JM, Kampinga GA, et al. Efficacy of Antibiotic Suppressive Therapy in Patients with a Prosthetic Joint Infection. J Bone Jt Infect. 2017;2(2):77–83.
- Prendki V, Ferry T, Sergent P, et al. Prolonged suppressive antibiotic therapy for prosthetic joint infection in the elderly: a national multicentre cohort study. Eur J Clin Microbiol Infect Dis. 2017 Sep;36(9):1577–1585.
- Pradier M, Robineau O, Boucher A, et al. Suppressive antibiotic therapy with oral tetracyclines for prosthetic joint infections: a retrospective study of 78 patients. Infection. 2018 Feb;46(1):39–47.
- Tan TL, Kheir MM, Rondon AJ, et al. Determining the Role and Duration of the "Antibiotic Holiday" Period in Periprosthetic Joint Infection. J Arthroplasty. 2018 Sep;33(9):2976–2980.
- Burnett RS, Aggarwal A, Givens SA, et al. Prophylactic antibiotics do not affect cultures in the treatment of an infected TKA: a prospective trial. Clin Orthop Relat Res. 2010 Jan;468(1):127–134.
- Bedencic K, Kavcic M, Faganeli N, et al. Does Preoperative Antimicrobial Prophylaxis Influence the Diagnostic Potential of Periprosthetic Tissues in Hip or Knee Infections? Clin Orthop Relat Res. 2016 Jan;474(1):258–264.
- Taha M, Abdelbary H, Ross FP, et al. New Innovations in the Treatment of PJI and Biofilms-Clinical and Preclinical Topics. Curr Rev Musculoskelet Med. 2018 Sep;11(3):380–388.
- Orthopedics Today. What is on the horizon for periprosthetic joint infection? 2012 [cited 2019. September 24,]. Available from: https://www.healio.com/orthopedics/infection/news/print/orthopedics-today/%7B8d534714-b381-48a3-85be-dfb396385194%7D/what-is-on-the-horizon-for-periprosthetic-joint-infection
- Giersing BK, Dastgheyb SS, Modjarrad K, et al. Status of vaccine research and development of vaccines for Staphylococcus aureus. Vaccine. 2016 Jun 3;34(26):2962–2966.
- Gupta TT, Karki SB, Matson JS, et al. Sterilization of Biofilm on a Titanium Surface Using a Combination of Nonthermal Plasma and Chlorhexidine Digluconate. Biomed Res Int. 2017;2017:6085741.
- Wang M, Tang T. Surface treatment strategies to combat implant-related infection from the beginning. J Orthop Translat. 2019 Apr;17:42–54.
- Greimel F, Scheuerer C, Gessner A, et al. Efficacy of antibiotic treatment of implant-associated Staphylococcus aureus infections with moxifloxacin, flucloxacillin, rifampin, and combination therapy: an animal study. Drug Des Devel Ther. 2017;11:1729–1736.
- Nodzo SR, Tobias M, Ahn R, et al. Cathodic Voltage-controlled Electrical Stimulation Plus Prolonged Vancomycin Reduce Bacterial Burden of a Titanium Implant-associated Infection in a Rodent Model. Clin Orthop Relat Res. 2016 Jul;474(7):1668–1675.
- Lehar SM, Pillow T, Xu M, et al. Novel antibody-antibiotic conjugate eliminates intracellular S. aureus. Nature. 2015 Nov 19;527(7578):323–328.
- Lehoux D, Ostiguy V, Cadieux C, et al. Oritavancin Pharmacokinetics and Bone Penetration in Rabbits. Antimicrob Agents Chemother. 2015 Oct;59(10):6501–6505.
- Fernandez J, Greenwood-Quaintance KE, Patel R. In vitro activity of dalbavancin against biofilms of staphylococci isolated from prosthetic joint infections. Diagn Microbiol Infect Dis. 2016 Aug;85(4):449–451.
- Liu Y, Busscher HJ, Zhao B, et al. Surface-Adaptive, Antimicrobially Loaded, Micellar Nanocarriers with Enhanced Penetration and Killing Efficiency in Staphylococcal Biofilms. ACS Nano. 2016 Apr 26;10(4):4779–4789.
- Chetoni P, Burgalassi S, Monti D, et al. Solid lipid nanoparticles as promising tool for intraocular tobramycin delivery: Pharmacokinetic studies on rabbits. Eur J Pharm Biopharm. 2016 Dec;109:214–223.
- Varshney AK, Kuzmicheva GA, Lin J, et al. A natural human monoclonal antibody targeting Staphylococcus Protein A protects against Staphylococcus aureus bacteremia. PLoS One. 2018;13(1):e0190537.
- Yang Y, Qian M, Yi S, et al. Monoclonal Antibody Targeting Staphylococcus aureus Surface Protein A (SasA) Protect Against Staphylococcus aureus Sepsis and Peritonitis in Mice. PLoS One. 2016;11(2):e0149460.
- Salmond GP, Fineran PC. A century of the phage: past, present and future. Nat Rev Microbiol. 2015 Dec;13(12):777–786.
- Schooley RT, Biswas B, Gill JJ, et al. Development and Use of Personalized Bacteriophage-Based Therapeutic Cocktails To Treat a Patient with a Disseminated Resistant Acinetobacter baumannii Infection. Antimicrob Agents Chemother. 2017 Oct;61(10).
- Briggs T, Blunn G, Hislop S, et al. Antimicrobial photodynamic therapy-a promising treatment for prosthetic joint infections. Lasers Med Sci. 2018 Apr;33(3):523–532.
- de Breij A, Riool M, Cordfunke RA, et al. The antimicrobial peptide SAAP-148 combats drug-resistant bacteria and biofilms. Sci Transl Med. 2018 Jan 10;10(423).
- de la Fuente-Nunez C, Reffuveille F, Mansour SC, et al. D-enantiomeric peptides that eradicate wild-type and multidrug-resistant biofilms and protect against lethal Pseudomonas aeruginosa infections. Chem Biol. 2015 Feb 19;22(2):196–205.
- Sebastian S, Malhotra R, Sreenivas V, et al. A Clinico-Microbiological Study of Prosthetic Joint Infections in an Indian Tertiary Care Hospital: Role of Universal 16S rRNA Gene Polymerase Chain Reaction and Sequencing in Diagnosis. Indian J Orthop. 2019 Sep-Oct;53(5):646–654.