Diagnosing periprosthetic joint infections: where to start, what to look for
Preview
While a periprosthetic joint infection (PJI) is more likely to occur within the first two years of implantation, any artificial joint is at an increased risk of developing infection over its lifetime. Diagnosing PJI is not always straightforward—patients may not have obvious symptoms. Detection of infection requires a multi-disciplinary approach characterized by good communication and weighted problem-solving based on the right information. What signs of infection should a clinician be on the lookout for? What combination of testing, imaging, and cultures is recommended? Part 2 of this article series examines the complexities of PJI diagnosis.
Periprosthetic joint infection (PJI) is a growing problem [1, 2]. Even if rates of infection hold steady, the simple fact that more joints are being replaced translates into a higher number of PJI cases [3–6]. The prevalence of multi-drug resistant PJIs is also a “worrisome” trend [7]. The microorganisms that tend to colonize artificial joints grow in biofilms that present a challenging host of problems in terms of diagnosis and treatment [8–10].
The complex nature of PJIs necessitates a multidisciplinary approach to coordinate decision making. The weighted evaluation of the information gathered at each step of diagnosis and treatment requires cross-disciplinary collaboration and communication [11]. See Part 1 of this article series for further elaboration of PJI risk factors and pre-, intra-, and postoperative infection prevention strategies. Part 3 examines treatment options for acute and chronic PJI.
In addition to the increased economic cost related to PJI [12], there is an often unacknowledged personal cost for surgeons who may feel responsible. In qualitative telephone interviews with orthopedic surgeons, Mallon et al chronicled “a significant emotional impact on surgeons who report a collective sense of devastation and personal ownership, even though prosthetic joint infection cannot be fully controlled for.” [13]
Most common pathogens in PJI
A 2014 study on the most common PJI pathogens compared 898 cases from an infection referral center in Germany to 772 cases at a similar institution in the US. A higher number of virulent and resistant organisms were identified as the source of infection in the US center. The organisms that were identified were: coagulase-negative Staphylococcus, Staphylococcus aureus, Streptococcus spp, Enterococcus spp, anaerobes, fungi, and mycobacteria. Additionally, they found polymicrobial and culture-negative infections [14]. Other causative agents are gram-negative bacteria (such as Klebsiella spp, Pseudomonas aeruginosa), and Cutibacterium spp [15].
This is a long list of suspects, each with unique biomarkers, culturing requirements, antibiotic susceptibility, and antibiotic dosing specifications and delivery pathways (intravenous vs oral). However, it is worth mentioning that there are also other infection-causing microorganisms rarely associated with PJI and this uncommonness may prolong or complicate their identification—they are not routinely tested for [16–19]. Testing for rare microorganisms has been recommended, particularly in persistent cases [18, 20].
Did you miss AO Recon’s webinar on periprosthetic joint infection (PJI)?
In June 2019, AO Recon gathered an online community of close to 200 surgeons for an interactive information session and Q&A led by Olivier Borens, Head of Septic Surgery and Head of Traumatology at the Centre Hospitalier Universitaire Vaudois (Lausanne, Switzerland) and chat moderator Andrej Trampuz, Infectious Diseases Consultant in Septic Surgery at Charité–Universitätsmedizin (Berlin, Germany) on the topic of infection after joint arthroplasty.
Biofilm: the orthopedic surgeon’s nemesis
When microbes grow as a biofilm they multiply into slimy, multi-layered, sometime multi-species, complex, synergistic communities. The microorganisms (bacteria and fungi) adhere to each other as well as surfaces, such as prosthetic joints, chemically communicating with each other. Bacteria in a biofilm share available nutrients and gain protection from hostile environmental threats such as antibiotics and the body’s immune defenses through a variety of strategies [21]. It is hypothesized that biofilm formation evolved as a survival strategy during the time of primitive Earth [22].
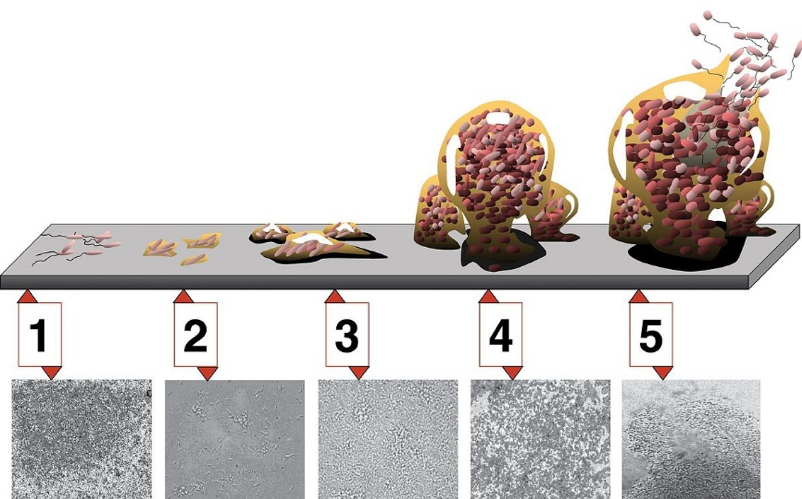
As they mature, biofilms, such as those formed by common PJI-causing Staphylococcus spp, can develop extracellular barriers, making it difficult for the body’s immune cells to penetrate, and even deactivate those that do manage to get through [23]. And most importantly in terms of PJI, biofilms are less sensitive to antibiotics via several inherent biological mechanisms [9]. Figure 1 highlights the intensifying complexity of a biofilm through its stages of maturity—it can be more straightforward to diagnosis and treat an infection before it forms a biofilm. Figure 1 also illustrates the persistence of biofilms, which when associated with PJI make them more difficult to predict, diagnose, and treat [24].
However, early PJI detection is not always possible and once you have a suspicion of infection there is no single test that can reliably be used to diagnose it, which means that a combination of diagnostic testing must be employed and interpreted [25–28].
Read the full article with your AO login
- Signs and symptoms
- Reconnaissance: know your adversary
- Imaging
- Laboratory testing
- Synovial fluid aspiration
- Microbiology
- Sonication
- Molecular methods
- Tissue histology
- Diagnose PJI if at least one of these criteria is satisfied
- What you’ve been waiting for
- Conclusion
- References
Additional AO resources on this topic
Access videos, tools, and other assets to learn more about this topic.
- Video: Updates in Infection Management after TKA
- Video: Infection After Joint Arthroplasty
- Further reading: Fracture-related infection: new consensus on diagnosis and treatment
- Upcoming events: AO Recon Course finder
Contributing experts
This series of articles was created with the support of the following specialists (in alphabetical order):

Olivier Borens
University Hospital Lausanne
Lausanne, Switzerland

Nora Renz
Inselspital—University Hospital Bern
Bern, Switzerland

Andrej Trampuz
Charité—University Medicine Berlin
Berlin, Germany
This issue was created by Word+Vision Media Productions, Switzerland.
References
- Inacio MCS, Paxton EW, Graves SE, et al. Projected increase in total knee arthroplasty in the United States—an alternative projection model. Osteoarthritis Cartilage. 2017 Nov;25(11):1797–1803.
- Carvalho RT, Lopes TL, Takano MI, et al. Evolution and projection of knee arthroplasties from 2003 to 2030 in the state of Sao Paulo. Rev Assoc Med Bras (1992). 2019 Aug 5;65(7):1001–1006.
- Kurtz SM, Lau E, Watson H, et al. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012 Sep;27(8 Suppl):61–65 e1.
- Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev. 2014 Apr;27(2):302–345.
- Ackerman IN, Bohensky MA, Zomer E, et al. The projected burden of primary total knee and hip replacement for osteoarthritis in Australia to the year 2030. BMC Musculoskelet Disord. 2019 Feb 23;20(1):90.
- Weaver AA, Hasan NA, Klaassen M, et al. Prosthetic joint infections present diverse and unique microbial communities using combined whole-genome shotgun sequencing and culturing methods. J Med Microbiol. 2019 Aug 28.
- Sebastian S, Malhotra R, Sreenivas V, et al. A Clinico-Microbiological Study of Prosthetic Joint Infections in an Indian Tertiary Care Hospital: Role of Universal 16S rRNA Gene Polymerase Chain Reaction and Sequencing in Diagnosis. Indian J Orthop. 2019 Sep-Oct;53(5):646–654.
- Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004 Oct 14;351(16):1645–1654.
- Jacqueline C, Caillon J. Impact of bacterial biofilm on the treatment of prosthetic joint infections. J Antimicrob Chemother. 2014 Sep;69 Suppl 1:i37–i40.
- Dibartola AC, Swearingen MC, Granger JF, et al. Biofilms in orthopedic infections: a review of laboratory methods. APMIS. 2017 Apr;125(4):418–428.
- Suren C, Feihl S, Querbach C, et al. Integrated IT Platform for Coordination of Diagnosis, Treatment, and Aftercare of Prosthetic Joint Infections. In Vivo. 2019 Sep-Oct;33(5):1625–1633.
- Scott RD, 2nd, Culler SD, Rask KJ. Understanding the Economic Impact of Health Care-Associated Infections: A Cost Perspective Analysis. J Infus Nurs. 2019 Mar/Apr;42(2):61–69.
- Mallon C, Gooberman-Hill R, Blom A, et al. Surgeons are deeply affected when patients are diagnosed with prosthetic joint infection. PLoS One. 2018;13(11):e0207260.
- Aggarwal VK, Bakhshi H, Ecker NU, et al. Organism profile in periprosthetic joint infection: pathogens differ at two arthroplasty infection referral centers in Europe and in the United States. J Knee Surg. 2014 Oct;27(5):399–406.
- PRO-IMPLANT Foundation. Pocket Guide to Diagnosis & Treatment of Periprosthetic Joint Infection (PJI). Version 8. 2019.
- Chenouard R, Hoppe E, Lemarie C, et al. A rare case of Prosthetic Joint Infection associated with Coxiella burnetii. Int J Infect Dis. 2019 Jul 30.
- Bhatnagar N, Poojary A, Maniar A, et al. Mycobacterium wolinskyi: A Rare Strain Isolated in a Persistent Prosthetic Knee Joint Infection: A Case Report. JBJS Case Connect. 2019 Aug 1.
- Rieber H, Frontzek A, Fischer M. Periprosthetic joint infection associated with Mycoplasma hominis after transurethral instrumentation in an immunocompetent patient. Unusual or underestimated? A case report and review of the literature. Int J Infect Dis. 2019 May;82:86–88.
- Kelly BC, Constantinescu DS, Foster W. Capnocytophaga canimorsus Periprosthetic Joint Infection in an Immunocompetent Patient: A Case Report. Geriatr Orthop Surg Rehabil. 2019;10:2151459318825199.
- Tsai Y, Chang CH, Lin YC, et al. Different microbiological profiles between hip and knee prosthetic joint infections. J Orthop Surg (Hong Kong). 2019 May-Aug;27(2):2309499019847768.
- Wikipedia. Biofilm. 2019 [cited 2019, September 17.]. Available from: https://en.wikipedia.org/wiki/Biofilm.
- Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the Natural environment to infectious diseases. Nature Reviews Microbiology. 2004 2004/02/01;2(2):95–108.
- Josse J, Valour F, Maali Y, et al. Interaction Between Staphylococcal Biofilm and Bone: How Does the Presence of Biofilm Promote Prosthesis Loosening? Front Microbiol. 2019;10:1602.
- El-Sayed D, Nouvong A. Infection Protocols for Implants. Clin Podiatr Med Surg. 2019 Oct;36(4):627–649.
- Gomez-Urena EO, Tande AJ, Osmon DR, et al. Diagnosis of Prosthetic Joint Infection: Cultures, Biomarker and Criteria. Infect Dis Clin North Am. 2017 Jun;31(2):219–235.
- Nodzo SR, Bauer T, Pottinger PS, et al. Conventional diagnostic challenges in periprosthetic joint infection. J Am Acad Orthop Surg. 2015 Apr;23 Suppl:S18–S25.
- Li C, Renz N, Trampuz A. Management of Periprosthetic Joint Infection. Hip Pelvis. 2018 Sep;30(3):138–146.
- Xing D, Ma X, Ma J, et al. Use of anti-granulocyte scintigraphy with 99mTc-labeled monoclonal antibodies for the diagnosis of periprosthetic infection in patients after total joint arthroplasty: a diagnostic meta-analysis. PLoS One. 2013;8(7):e69857.
- Izakovicova P, Borens O, Trampuz A. Periprosthetic joint infection: current concepts and outlook. EFORT Open Rev. 2019 Jul;4(7):482–494.
- Parker S, Key T, Hughes H, et al. The myth of surgical sterility: Bacterial contamination of knee arthroplasty drapes. 2018, February;98-B,(SUPP_23,).
- Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004 Apr 1;350(14):1422–1429.
- Signore A, Sconfienza LM, Borens O, et al. Consensus document for the diagnosis of prosthetic joint infections: a joint paper by the EANM, EBJIS, and ESR (with ESCMID endorsement). Eur J Nucl Med Mol Imaging. 2019 Apr;46(4):971–988.
- American College of Radiology. ACR Appropriateness Criteria® Imaging After Total Knee Arthroplasty. 2017. [cited 2017 September 21]. Available from: https://acsearch.acr.org/docs/69430/Narrative/
- Palestro CJ, Love C. Role of Nuclear Medicine for Diagnosing Infection of Recently Implanted Lower Extremity Arthroplasties. Semin Nucl Med. 2017 Nov;47(6):630–638.
- Gomes LSM. Early Diagnosis of Periprosthetic Joint Infection of the Hip-Current Status, Advances, and Perspectives. Rev Bras Ortop (Sao Paulo). 2019 Jul;54(4):368–376.
- Krupa K, Bekiesinska-Figatowska M. Artifacts in magnetic resonance imaging. Pol J Radiol. 2015;80:93–106.
- Verberne SJ, Raijmakers PG, Temmerman OP. The Accuracy of Imaging Techniques in the Assessment of Periprosthetic Hip Infection: A Systematic Review and Meta-Analysis. J Bone Joint Surg Am. 2016 Oct 5;98(19):1638–1645.
- Kwee TC, Kwee RM, Alavi A. FDG-PET for diagnosing prosthetic joint infection: systematic review and metaanalysis. Eur J Nucl Med Mol Imaging. 2008 Nov;35(11):2122–2132.
- Cozzi Lepri A, Del Prete A, Soderi S, et al. The identification of pathogens associated with periprosthetic joint infection in two-stage revision. Eur Rev Med Pharmacol Sci. 2019 Apr;23(2 Suppl):101–116.
- Shih LY, Wu JJ, Yang DJ. Erythrocyte sedimentation rate and C-reactive protein values in patients with total hip arthroplasty. Clin Orthop Relat Res. 1987 Dec(225):238–246.
- Yee DK, Chiu KY, Yan CH, et al. Review article: Joint aspiration for diagnosis of periprosthetic infection. J Orthop Surg (Hong Kong). 2013 Aug;21(2):236–240.
- Del Pozo JL, Patel R. Clinical practice. Infection associated with prosthetic joints. N Engl J Med. 2009 Aug 20;361(8):787–794.
- Renz N, Yermak K, Perka C, et al. Alpha Defensin Lateral Flow Test for Diagnosis of Periprosthetic Joint Infection: Not a Screening but a Confirmatory Test. J Bone Joint Surg Am. 2018 May 2;100(9):742–750.
- Yermak K, Karbysheva S, Perka C, et al. Performance of synovial fluid D-lactate for the diagnosis of periprosthetic joint infection: A prospective observational study. J Infect. 2019 Aug;79(2):123–129.
- Watanabe S, Kobayashi N, Tomoyama A, et al. Differences in Diagnostic Properties Between Standard and Enrichment Culture Techniques Used in Periprosthetic Joint Infections. J Arthroplasty. 2019 Aug 19.
- Drago L, Clerici P, Morelli I, et al. The World Association against Infection in Orthopaedics and Trauma (WAIOT) procedures for Microbiological Sampling and Processing for Periprosthetic Joint Infections (PJIs) and other Implant-Related Infections. J Clin Med. 2019 Jun 28;8(7).
- Hughes JG, Vetter EA, Patel R, et al. Culture with BACTEC Peds Plus/F bottle compared with conventional methods for detection of bacteria in synovial fluid. J Clin Microbiol. 2001 Dec;39(12):4468–4471.
- Li C, Ojeda-Thies C, Trampuz A. Culture of periprosthetic tissue in blood culture bottles for diagnosing periprosthetic joint infection. BMC Musculoskelet Disord. 2019 Jun 22;20(1):299.
- Renz N, Mudrovcic S, Perka C, et al. Orthopedic implant-associated infections caused by Cutibacterium spp. - A remaining diagnostic challenge. PLoS One. 2018;13(8):e0202639.
- Parvizi J, Erkocak OF, Della Valle CJ. Culture-negative periprosthetic joint infection. J Bone Joint Surg Am. 2014 Mar 5;96(5):430–436.
- Huang Z, Wu Q, Fang X, et al. Comparison of culture and broad-range polymerase chain reaction methods for diagnosing periprosthetic joint infection: analysis of joint fluid, periprosthetic tissue, and sonicated fluid. Int Orthop. 2018 Sep;42(9):2035–2040.
- Hoiby N, Bjarnsholt T, Moser C, et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect. 2015 May;21 Suppl 1:S1–25.
- Trampuz A, Piper KE, Jacobson MJ, et al. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007 Aug 16;357(7):654–663.
- Kummer A, Tafin UF, Borens O. Effect of Sonication on the Elution of Antibiotics from Polymethyl Methacrylate (PMMA). J Bone Jt Infect. 2017;2(4):208–212.
- Borens O, Yusuf E, Steinrucken J, et al. Accurate and early diagnosis of orthopedic device-related infection by microbial heat production and sonication. J Orthop Res. 2013 Nov;31(11):1700–1703.
- Morgenstern C, Cabric S, Perka C, et al. Synovial fluid multiplex PCR is superior to culture for detection of low-virulent pathogens causing periprosthetic joint infection. Diagn Microbiol Infect Dis. 2018 Feb;90(2):115–119.
- Lausmann C, Zahar A, Citak M, et al. Are There Benefits In Early Diagnosis Of Prosthetic Joint Infection With Multiplex Polymerase Chain Reaction? J Bone Jt Infect. 2017;2(4):175–183.
- Portillo ME, Salvado M, Sorli L, et al. Multiplex PCR of sonication fluid accurately differentiates between prosthetic joint infection and aseptic failure. J Infect. 2012 Dec;65(6):541–548.
- Saeed K, Ahmad-Saeed N. The impact of PCR in the management of prosthetic joint infections. Expert Rev Mol Diagn. 2015;15(7):957–964.
- Achermann Y, Vogt M, Leunig M, et al. Improved diagnosis of periprosthetic joint infection by multiplex PCR of sonication fluid from removed implants. J Clin Microbiol. 2010 Apr;48(4):1208–1214.
- Ballenghien M, Faivre N, Galtier N. Patterns of cross-contamination in a multispecies population genomic project: detection, quantification, impact, and solutions. BMC Biol. 2017 Mar 29;15(1):25.
- Bauer TW, Parvizi J, Kobayashi N, et al. Diagnosis of periprosthetic infection. J Bone Joint Surg Am. 2006 Apr;88(4):869–882.
- Tohtz SW, Muller M, Morawietz L, et al. Validity of frozen sections for analysis of periprosthetic loosening membranes. Clin Orthop Relat Res. 2010 Mar;468(3):762–768.
- Bemer P, Leger J, Milin S, et al. Histopathological Diagnosis of Prosthetic Joint Infection: Does a Threshold of 23 Neutrophils Do Better than Classification of the Periprosthetic Membrane in a Prospective Multicenter Study? J Clin Microbiol. 2018 Sep;56(9).
- Muller M, Morawietz L, Hasart O, et al. [Histopathological diagnosis of periprosthetic joint infection following total hip arthroplasty : use of a standardized classification system of the periprosthetic interface membrane]. Orthopade. 2009 Nov;38(11):1087–1096.
- Krenn V, Morawietz L, Perino G, et al. Revised histopathological consensus classification of joint implant related pathology. Pathol Res Pract. 2014 Dec;210(12):779–786.