SYMPHONY OCT Compartment 2
Richard Bransford, Maarten Spruit
The SYMPHONY™ OCT System is an enhanced set of instruments and implants for posterior fixation of the occipital-cervical junction, subaxial spine, and cervico-thoracic junction. Compartment 1 of the SYMPHONY OCT system was launched in November 2019. SYMPHONY Compartment 1 has recently been supplemented by Enhanced and Innovative elements (Compartment 2) to further address surgical needs and avoid pitfalls in a complex cervical surgery. Launched in early 2021, the Compartment 2 components include MULTIPOINT™ SECURE to augment lateral mass screws, reduction screws, advanced connector and various rod designs, a short drill guide, and polydriver. This addition builds on the advanced features already found in Compartment 1, which took the best of Synthes Synapse and DePuy Mountaineer.
Clinical Need
Occipital-Cervical-Thoracic (OCT) Systems have been developed to provide posterior correction and stabilization of spinal segments as an adjunct to fusion from the occiput to the thoracic spine. The current generation of OCT systems address the clinical needs for open posterior approach for numerous pathologies to include degenerative conditions, trauma, tumors, deformities, and infections.
Fixation
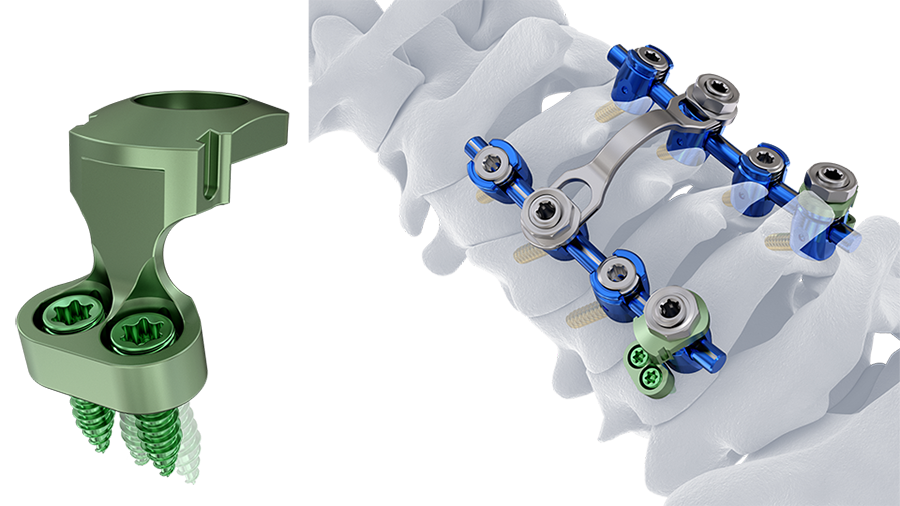
By optimizing and improving instruments and implants for posterior cervical fusion (PCF) following FATE principle (fixation, alignment, targeting, and extension), Compartment 2 addresses surgical and technical concerns in an aging population with sub-optimal bone quality as well as in patients who may require stronger constructs meeting the indications for spinal fusion [1–3]. There are also an increasing number of patients requiring extensions from prior posterior instrumented fusions. Screw fixation failure is another frequently reported issue (up to 5.2%) in PCF, and patients with sub-optimal bone quality may be at a greater risk [3, 4]. More robust fixation of lateral mass screws (C3-C7) is now provided with MULTIPOINT SECURE (Fig 1).
A significant potential complication associated with lateral mass screws is loss of fixation. Market research reveals that 95% of surgeons have observed a lateral-mass screw pullout or screw loosening [5]. The supplemental use of MULTIPOINT SECURE adds additional points of fixation, as the load can be shared across additional points of fixation via a locking plate. Reinforcing a lateral mass screw with MULTIPOINT SECURE with additional screws, increases the resistance to screw pullout by an average of 48.2% compared with standalone lateral mass screws [5].
Alignment
Improvements in cervical alignment after deformity correction are correlated with improvement in HRQoL [6]. Market research indicates limited surgeon satisfaction in the ability to achieve alignment targets with the current instrumentation systems [5].
A single 4.0 mm SYMPHONY OCT System rod achieves bio-mechanical equivalence to a 3.5–5.5 mm construct in range-of-motion testing. Utilizing a single system for long cervico-thoracic constructs may reduce procedure complexity and facilitate alignment correction.
The SYMPHONY OCT System offers 3.5 mm and 4.0 mm CoCr rods, both demonstrated to be stiffer and stronger than titanium equivalents [5]. Stronger and stiffer constructs may support surgeons in achieving regional cervical alignment targets.
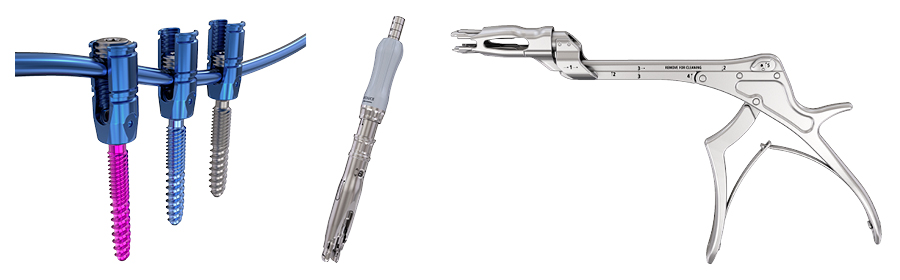
Besides the Reduction Towers and the Kerrison Reducer, cannulated Reduction Screws are now offered in the system with Compartment 2 (Fig 2). Reduction screws and Reduction Towers were designed to simplify rod capture in complex deformity surgeries and allow for a more harmonious spinal force distribution and prevention of screw pullout [5].
Targeting and Navigation
According to DePuy Synthes data [5] 91% of surgeons have reported feeling less than satisfied with their screw placement’s initial trajectory. Cannulated screws provide a less invasive technique for the insertion of cervical pedicle screws. The use of cannulated screws has been shown to decrease perforation and complication rates in adolescent idiopathic scoliosis spine surgery [7].The SYMPHONY OCT System instruments are navigation compatible and are offered to reduce the risk of screw breach and malpositioning. Rates of pedicle perforations in the cervical spine have been shown to be lower with computer-assisted surgery compared with conventional techniques (3.0% vs 8.6%) [8]. Furthermore, navigated instrumentation may reduce radiation exposure to the user, providing a safer environment for surgeons [9–11].
With Compartment 2, key instruments can now be navigated with navigation arrays and adaptors (Fig 3). There are two Universal Navigation Adaptor Sets (UNAS)—one set that can be used with a Medtronic SureTrak(R) II array and be manually calibrated to the Medtronic Stealth Station navigation system. The other set works directly with Brainlab, and includes pre-calibrated instruments and new, aligned Brainlab Software that can substantially improve the surgical workflow. The cannulated screws provide a potentially less invasive technique for the insertion of cervical pedicle screws.
Extension and Revision
Posterior cervical fusion revisions may involve the removal, replacement, and extension of existing constructs that can undeniably drive-up revision cost and time. Extending rather than replacing existing constructs may help to save revision-related costs. Market research has shown limited surgeon satisfaction with the ability to extend constructs, and roughly 56% of surgeons state that they currently have difficulty inserting connectors [5].
The SYMPHONY OCT System offers comprehensive rod and connector options that were designed to match patient anatomy and accept multiple rod sizes (3.5– 6.35 mm) providing the ability to connect to existing systems.
Compartment 2 now has been supplemented with Advanced Connectors designed to accept multiple rod sizes, accommodate multiple systems, and reduce procedure complexity. Currently it also offers Offset Rods (Fig 4).
The SYMPHONY OCT System instruments have been enhanced with ergonomic instruments which facilitate the new features of Polyscrew, percutaneous and interconnecting implants (MULTIPOINT SECURE, Reduction Connector, Top-Loading Rod Connector and Hooks) (Fig 5).
For more information and insight on the instruments and other features check the Surgical Technique Guide (SYMPHONY OCT System).
Conclusion
Compartment 2 of the SYMPHONY OCT System is an AO TC approved extension of Compartment 1, resulting in a state-of-the-art posterior cervical instrumentation system with features that address surgical needs and promote improved patient outcomes.
References
1. Salzmann SN, Derman PB, Lampe LP et al. Cervical spinal fusion: 16-year trends in epidemiology, indications, and in-hospital outcomes by surgical approach. World Neurosurg. 2018 May;113 e280–e295.
2. Safaee MM et al. Neurosurg Open. 2020;1(2).
3. Reitman CA, Lyndon Nguyen, Fogel GR. Biomechanical evaluation of relationship of screw pullout strength, insertional torque, and bone mineral density in the cervical spine. J Spinal Disord Tech. 2004 Aug;17(4):306–311.
4. Coe JD, Vaccaro AR, Dailey A, et al. Lateral mass screw fixation in the cervical spine: a systematic literature review. J Bone Joint Surg Am. 2013 Dec 4;95 (23): 2136–2143.
5. DePuy Synthes SYMPHONY System Internal Data on File— ADAPTIV 103584004. Unpublished.
6. Tang JA, Scheer JK, Smith JS, et al. The impact of standing regional cervical sagittal alignment on outcomes in posterior cervical fusion surgery. Neurosurgery. 2012 Sep;71(3):662–669; discussion 669.
7. Yilar S. Comparison of the accuracy of cannulated pedicle screw versus conventional pedicle screw in the treatment of adolescent idiopathic scoliosis: A randomized retrospective study. Medicine (Baltimore). 2019 Mar;98 (10):e14811.
8. Richter M, Cakir B, Schmidt R. Cervical pedicle screws: conventional versus computer-assisted placement of cannulated screws. Spine (Phila Pa 1976). 2005 Oct 15;30(20):2280–2287.
9. Shin BJ, James AR, Njoku IU, et al. Pedicle screw navigation: a systematic review and meta-analysis of perforation risk for computer-navigated versus freehand insertion. J Neurosurg Spine. 2012 Aug;17(2):113–122.
10. Kim CW, Lee YP, Taylor W, et al. Use of navigation-assisted fluoroscopy to decrease radiation exposure during minimally invasive spine surgery. Spine J. 2008 Jul-Aug;8(4):584–590.
11. Kraus MD, Krischak G, Keppler P, et al. Can computer-assisted surgery reduce the effective dose for spinal fusion and sacroiliac screw insertion? Clin Orthop Relat Res. 2010 Sep;468(9):2419– 2429.
Clinical case using MULTIPOINT SECURE provided by Richard Bransford, Harborview Medical Center, Seattle, Washington, USA.
Case 1
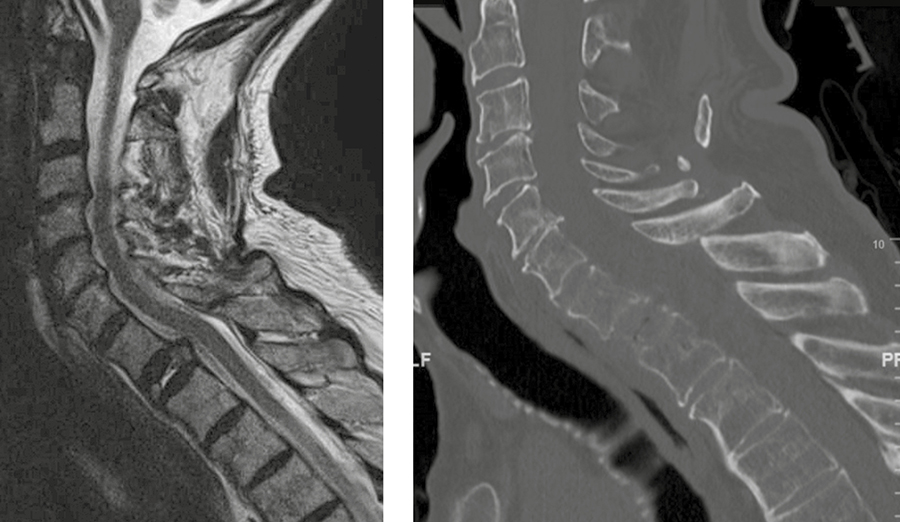
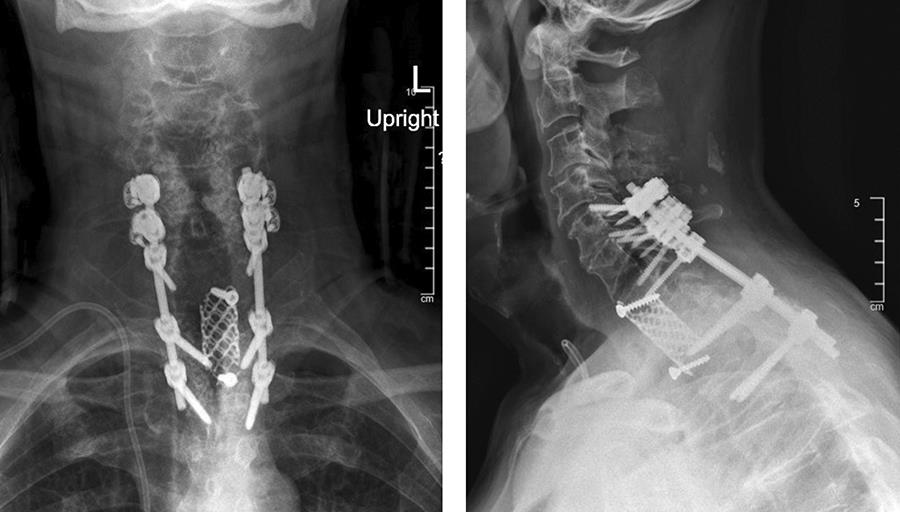
A 73-year-old man with a history of renal cell carcinoma presented with progressive bilateral upper extremity paresthesia/numbness, left upper extremity weakness, and upper back pain with a newly identified T1 pathological fracture (Fig 6).
The patient was taken to the operating room and underwent:
· Anterior T1 corpectomy and decompression of tumor and compressive material.
· Surgical reconstruction with DePuy Synthes SYNMESH cage and restoration of height. Given manubrium and angle of access, an anterior plate was not applied.
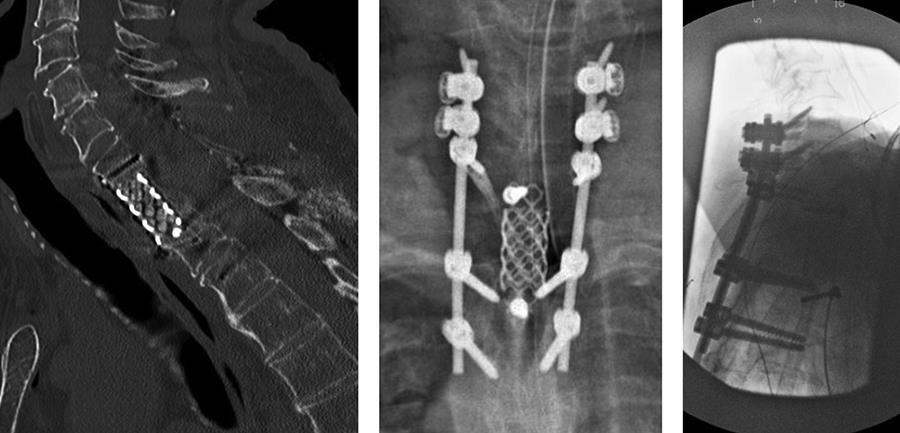
· C5-T3 posterior instrumentation with the DePuy Synthes SYMPHONY OCT System. Given the patient’s suboptimal bone quality, C5 and C6 lateral mass screws were reinforced with two MULTIPOINT SECURE screws placed bilaterally for an additional eight screws (Fig 7).
The patient had immediate relief of the radicular symptoms postoperatively. Three months postoperatively (Fig 8), he had no neck pain and was anxious to return to daily exercise at the gym. He had maintained resolution of his radiculopathy and was satisfied with the outcome.
Symphony—Occipito-Cervico-Thoracic System
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.