PFNA Asian Size (PFNA II)
Unstable trochanteric fractures, mainly due to osteoporosis, are a surgical challenge worldwide. However, significant regional variations are apparent in both the size and the characteristics of the problem. For example, it is known that East-Asians have different geometric proportions to Caucasians, resulting in complications due to a mismatch when using standard implants.
The design of the PFNA was developed based on the anatomy of the Caucasian population. For the average Asian patient, the fit of these implants is not optimal. Therefore, cadaver studies using CT scans were performed to adapt the design of the PFNA to the anatomy of the Japanese and other Asian population.
Features of the Proximal Femoral Nail Antirotation (PFNA) Asia
The Proximal Femoral Nail Antirotation (PFNA), with its helical blade concept, is a state-of-the-art treatment method for proximal femoral fractures, providing rotational and angular stability with one single element. The intramedullary nail acts as an internal splint that controls but does not prevent micromovements of the bone fragments. The PFNA provides relative stability, leading to indirect healing through callus formation.
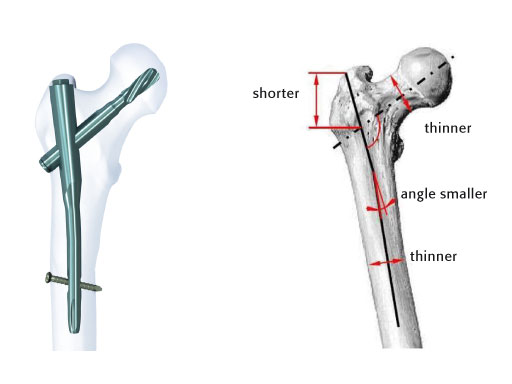
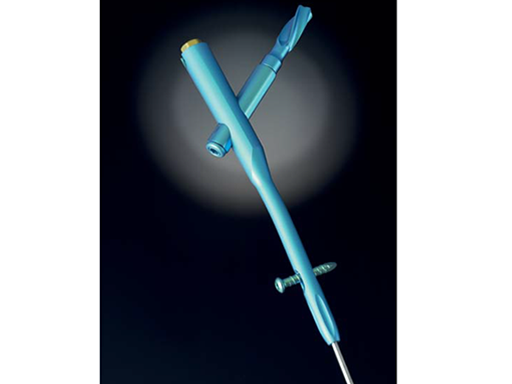
To help cope with the differences described above, an Asian version of the existing Proximal Femoral Nail Antirotation (PFNA) was developed and introduced into the market. The PFNA Asia (also known as PFNA II) (Fig 1) has a better fit to the smaller trochanteric area and narrower intramedullary canal of the Asian population. It features a lateral flat surface which makes insertion easier and lowers the pressure on the lateral cortex. The bend has been reduced to 5° versus 6° compared to the PFNA. The proximal diameter has been reduced to 16.5 mm (versus 17.0 mm). Furthermore, the spiral blade diameter has been reduced to 10.3 mm.
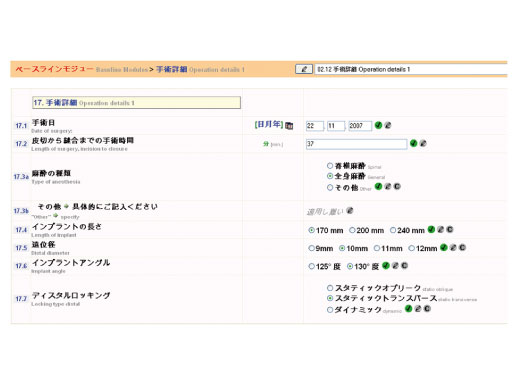
The PFNA Asian size comes in four different distal diameters from 9–12 mm, each in three lengths (170, 200, and 240 mm*). The long PFNA Asian size (260–340 mm, with 20 mm increments) is available in two distal diameters of 9 and 10 mm. Additional lengths of 340–420 mm in 40 mm increments are available with a distal diameter of 10 mm. The blade is available in lengths of 75–120 mm in steps of 5 mm. Two versions of Caput Collum Diaphyseal (CCD) angles of 125° or 130° are available. The nail is made out of titanium aluminum niobium (TAN) and sterile packaged.
In addition, some selected instruments have also been specifically designed for the smaller stature of the population and to the needs of the Asian surgeons.
The PFNA Asian size is indicated for pertrochanteric fractures (Müller AO Classification 31-A1 and 31-A2), intertrochanteric fractures (31-A3), and high subtrochanteric fractures (32-A1). The long version is indicated for low and extended subtrochanteric fractures, ipsilateral trochanteric fractures, combination fractures in the proximal femur, and pathological fractures.
*The 240 mm nail comes in two different distal diameters 9 and 10 mm only.
PFNA/PFNA-II Blade Extraction Set
The PFNA/PFNA-II blade extraction set is a special set containing instruments for the extraction of the PFNA/PFNA-II blade and received its AO TC approval in November 2009. Usually, the blade is removed by the extraction screw for PFNA blade. But in certain cases, especially in younger patients with good bone quality, the PFNA/PFNA-II blade may not be removable with the standard surgical technique and instrumentation. The new extraction set offers special instrumentation for potential issues such as damaged recess of blade, extracted end cap of blade, broken screw, extracted sleeve of blade, and parts of broken instruments in end part of blade.
Proximal Femoral Nail Removal Set
The proximal femoral nail removal set is a comprehensive set containing instruments for the removal of all proximal femoral nails as the trochanteric fixation nail (TFN short, standard, and long), the proximal femoral nail (PFN extra small, small, standard, and long), the proximal femoral nail antirotation (PFNA extra small, small, standard, and long), and PFNA-II (extra small, small, standard, and long). It received AO TC approval in November 2009.
The proximal femoral nail removal set contains general instruments for implant removal as well as all system-specific extraction instruments for the different proximal femoral nails. This prevents abort of surgery due to wrong set order or wrong identification of nailing system and avoids delays caused by missing or incorrect instruments.
Case 1: Woman with fall injury
Cases provided by Takeshi Sawaguchi, Toyama, Japan.
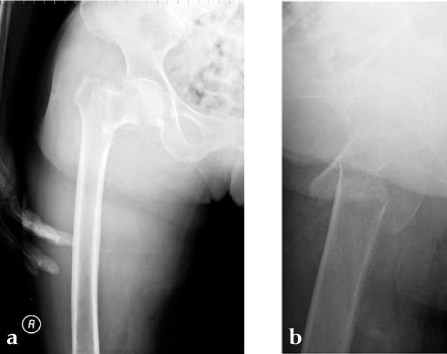
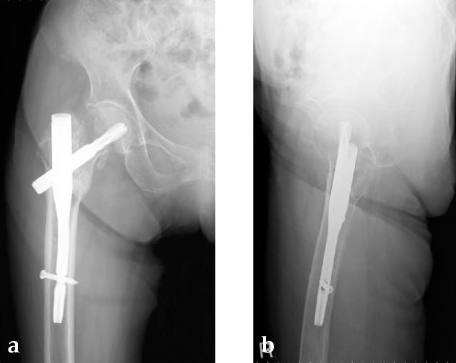
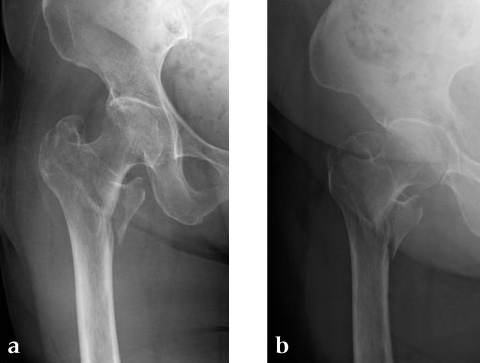
A 78-year-old Japanese patient fell while at her home and sustained an AO 31-A2.1 injury (see Fig 5).
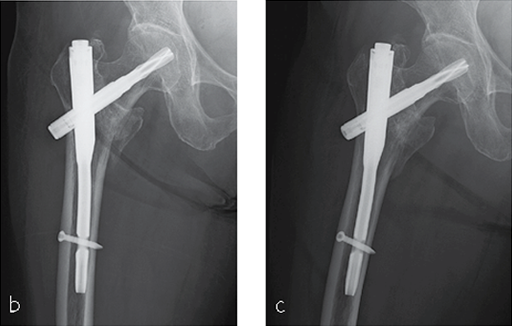
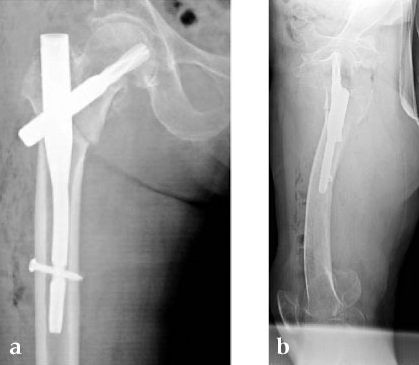
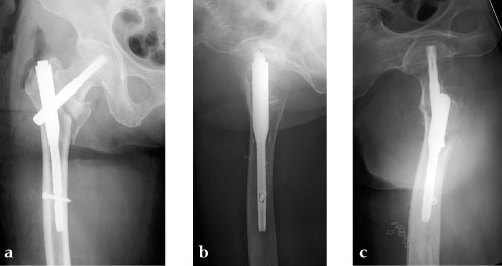
She was operated on the next day and fixed with a PFNA Asia (size: extra small, angle 130 degrees, distal diameter: 9 mm, blade length 100 mm) (Fig 6a). Good reduction and stable fixation were obtained. Full weight-bearing gait was allowed on the second postoperative day. There was an uneventful postoperative course, and good union was obtained at three months after surgery (Fig 6b).
Case 2: Elderly patient with fall injury
Case provided by Takeshi Sawaguchi, Toyama, JP
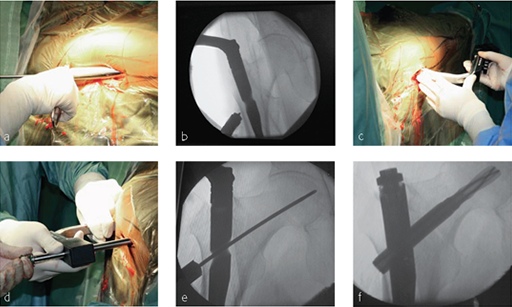
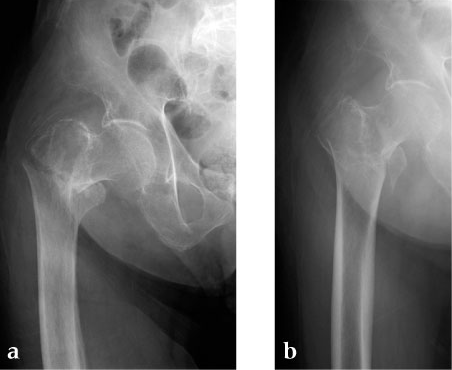
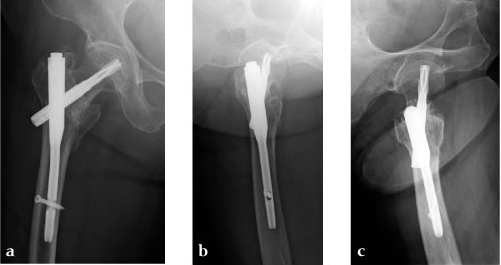
An 85-year-old Japanese woman fell down at her home. She sustained an AO 31-A2.1 fracture. The images indicate various stages of the operation, using the PFNA Asia.
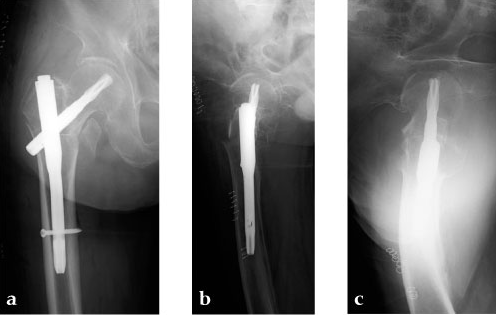
Case 3: 90-year-old male, injured by fall down.
Case provided by Toru Sato, Okayana, JP
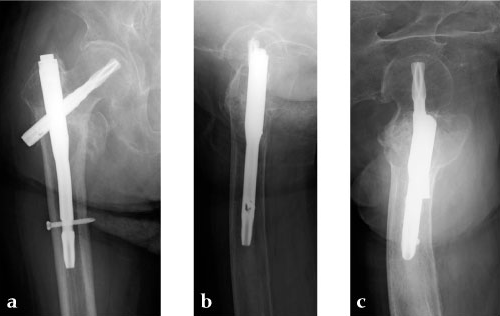
Case 4: 77-year-old male.
Case provided by Takeshi Sawaguchi, Toyama, JP
78-year-old female.
Case provided by Takeshi Sawaguchi, Toyama, JP
Japanese Proximal Femoral Nail (Antirotation) Study - The AOs First Completely Bilingual Study
AO Clinical Investigation (AOCID) has, in cooperation with Japanese colleagues, initiated the first multicenter study in orthopedic trauma in Japan in 2008. Among Japanese patients aged 65 years and older with proximal femoral fractures, the proportion of those aged 90 years and older has increased over the last decades from 3% in 1983 to nearly 20% in 2004. One reason for this is the increasing number of patients with osteoporosis in the older population. Compared to other ethnic groups, Asian people have the lowest bone mass.
Study background
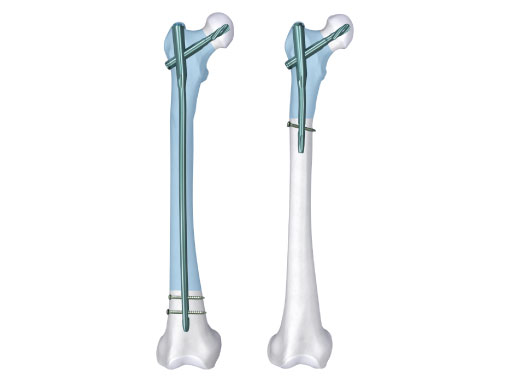
The idea for the study came from the Asian Trauma Working Group (ASWG) because Asian people have different geometric proportions of the femoral bone compared to European or American populations. For example, studies have shown that Asian women have shorter femoral necks, smaller femoral neck angles, and a more anterior bowing of the shaft than white Americans. The new PFNA Asia has specially adapted sizes and geometry to better suit the local population. It boasts a flat lateral side, higher bending point of the nail resulting in a straighter tip point, a smaller medial-lateral bending of 5, and adapted surgical instruments. Japanese doctors were the driving force behind the development of the PFNA Asia and are pivotal to this study. In addition to the principal clinical investigator, Dr Takeshi Sawaguchi, Drs Tadashi Tanaka, Yasuhiro Nanri, and Atsushi Sakurai are also central to the study's success.
Although original PFNA have been in clinical use since 2005 in Japan, no data on the rate of mismatch are available yet. Therefore, one of the study's goals is to determine whether potential mismatch exists between the new PFNA Asia and Japanese patients, and whether both Japanese patients and surgeons needs will be met.
Objectives of the study
The primary objective of this prospective multicenter case series is to assess any fracture fixation complication and revision rates during the clinical use of the proximal femoral nail antirotation Asia (PFNA Asia) for the treatment of unstable, closed trochanteric fractures, classified according to the Müller AO Classification type 31-A2 or 31-A3. Fracture fixation complications include any bone/fracture- or implant/surgery-related event.
In addition to the assessment of potential mismatches of femoral medullary cavity and the PFNA Asia at the femoral great trochanter (lateral to blade insertion point), the middle medial portion and distal end of the nail are investigated: soft tissue, wound or general complications, prognostic factors for the occurrence of complications (eg, bone mineral density, dementia), length of hospital stay, walking ability, capacity to return to pre-injury status, quality of life, mortality, anatomical restoration (clinically and radiographically), length of surgery (skin to skin time) and operative handling of the implant and the instrumentation.
The follow-up schedule applying to this study includes initial hospitalization, as well as follow-up visits at 6 weeks, 12 weeks, 6 months and 12 months (these are the defined time intervals). The final evaluation of radiographs and complications will be performed by a study review board at the end of the study.
First completely bilingual study
Every document in the study is written in both Japanese and English. For example, every question on each case report form is asked in Japanese and English. The aim of this is to ease the filling out of the case report forms for Japanese colleagues. Additionally, for the first time, the OpVerdi database has been completely created in both languages. This naturally created a lot of work for the AOs team of computer specialists due to the different characters used in both languages. The key to success for the planning phase of this study was the close collaboration between the principle clinical investigator (PCI) in Japan and the responsible AOCID study manager, despite a distance of more than 10,000 kilometers between them.
Study conduct
The clinical investigation was performed in 20 Japanese hospitals over a three year period. The study population was 176 patients aged 65 years and older with a radiologically-confirmed, unstable, closed trochanteric fracture, sustained seven days or less before primary fixation treatment with the PFNA Asia.
Preliminary results and conclusion
Patient functional outcomes and health-related quality of life were similar between the pre-injury and final follow-up evaluations, and surgeons were highly satisfied with the implant and its handling. There is also a large societal benefit to enabling patients of advanced age that have sustained an unstable trochanteric fracture to return to their preinjury residential status.
The preliminary results from this study highlight the importance of having implants designed for older Asian patients available to surgeons in the region.
Full text and further information
Sawaguchi T, Sakagoshi D, Shima Y, Ito T, Goldhahn S. Do design adaptations of a trochanteric nail make sense for Asian patients? Results of a multicenter study of the PFNA-II in Japan, Injury, Volume 45, Issue 10, 2014, Pages 1624-1631, ISSN 0020-1383,
More information on the study methodology is available on www.ClinicalTrials.gov under the identifying number: NCT00873548.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.