Craniofacial Distraction System
Since its introduction to craniomaxillofacial surgery in the early 1990s, distraction osteogenesis (DO) has become an increasingly popular technique in the correction of congenital and posttraumatic deformities of the mandible by gradually initiating and controlling new bone growth and expansion of the surrounding soft tissues. Most common amongst the numerous congenital craniofacial anomalies which result in an undersized or retrusive mandible are Treacher Collins syndrome, Pierre Robins Sequence (PRS), Nagers syndrome and hemifacial microsomia. Associated with, and directly related to the bony hypoplasis are serious airway, nutrition and sleeping disorders, which can often be life threatening.
Distraction osteogenesis has become the subject of AO events such as the Advanced Course in Hong Kong in November 2007 or the first Distraction Experts’ Symposium in Naples, Italy, in September 2007, where craniomaxillofacial surgeons from eight different countries discussed their experience using different distraction systems with a focus on the potential improvement of existing systems.
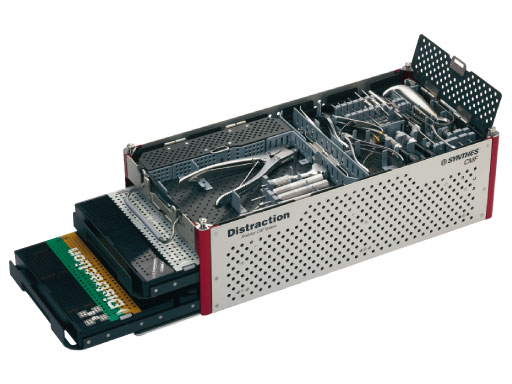
The clinical feedback obtained over the past years from surgeons with experience in DO has led to the development of the new CMF distraction system (Fig 1).
> Please note that the new CMF Distractor has received approval in 2019.
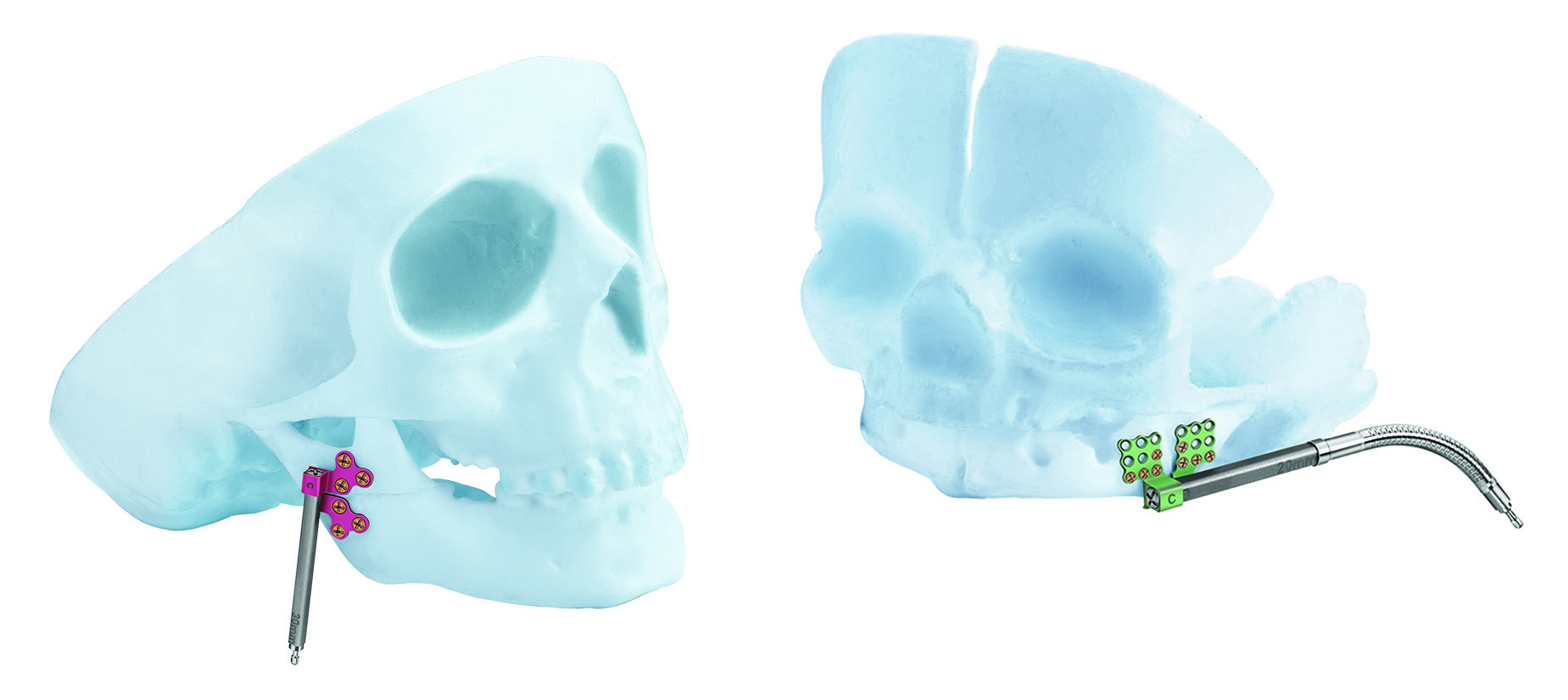
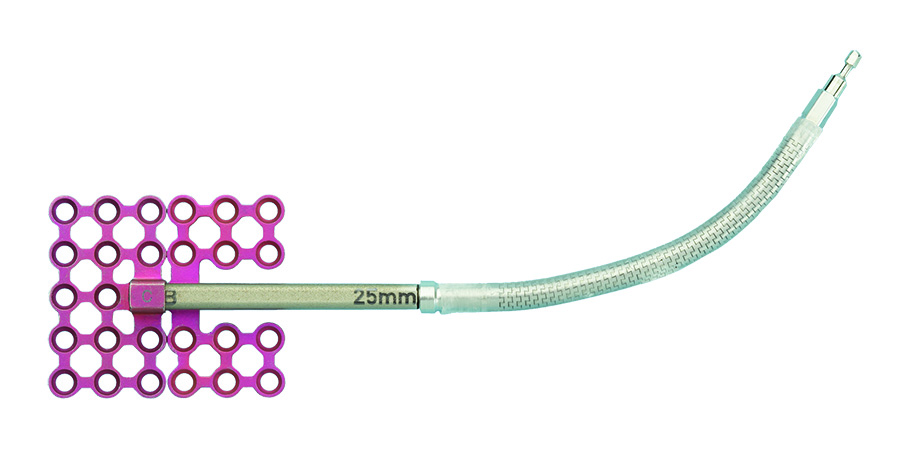
A modular family of internal distraction osteogenesis devices that are used to gradually lengthen the mandibular body and ramus. Each device, when assembled, is comprised of a distractor body, two footplates, and a machine screw to secure the assembly. The system also includes optional activation arms, which can be attached to the activation end of the device to move the point of activation to an area accessible by the activation instrument. Additionally this arm can be removed during the phase of consolidation (Fig 2).
The new CMF distraction system includes two configurations of distractor bodies: The AB (center translating) distractor and the BC (end translating) distractor. Both distractor bodies have an internal threaded lead screw, which engages the threaded footplates.
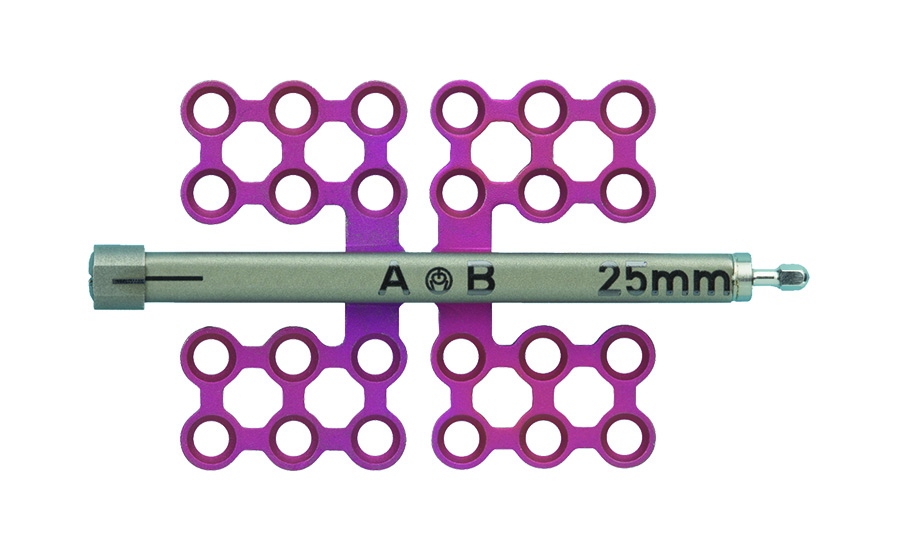
The AB distractor (Fig 3) is designed for placement on both sides of the osteotomy, ie, at the anterior side or midbody of the mandible. In contrast the BC distractor is designed to be used when most of the distractor body can be placed on one side of the osteotomy, ie, at the posterior body or ramus for advancement of the mandibular body, while leaving the temporomandibular segment motionless.
The AB distractor works with two footplates marked as "A" and "B". Both footplates are initially threaded to the center of the lead screw. When the device is activated, the two footplates move away from each other in opposite directions along the lead screw.
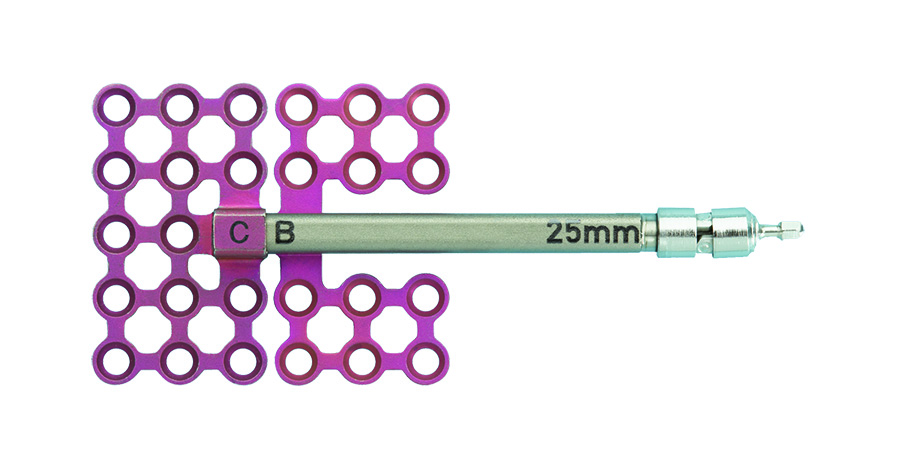
The BC distractor (Fig 4) uses a "B" footplate which is threaded onto the lead screw and a nonthreaded "C" footplate, which is attached over the end of the distractor body with a machine screw. When the device is activated, the "B" footplate moves along the lead screw, separating it from the "C" footplate, which remains motionless.
The AB and BC style distractor bodies are offered either with or without a universal joint attached on the activation end of the distractor body. The universal joint aids in the attachment of activation arms. All of the distractor bodies are 3.4 mm in diameter and are available in various lengths from 1530 mm.
The system includes removable activation arms, which are available in various lengths in a rigid or flexible design (Fig 5). The extension arms allow the devices point of activation to be located away from the distractor body to an area (either intraoral or percutaneous port) that is easily accessible by the activation instrument.
The extension arms can be removed once the distraction phase is completed without the need for a second surgical procedure by disengaging the outer sleeve from the inner sleeve using a removal instrument. The distractor can then be buried in the soft tissue. The possibility of inflammation or infection at the transcutaneous or transmucosal portal will be reduced, as is the social inconvenience caused by protusion of the extension arm.
Steps of a normal placement procedure are summarized as follows:
- Standard surgical incision (intraoral or submandibular)
- Assembly and fitting of the distractor
- Standard surgical osteotomy and bone mobilization
- Fixation of the distractor
- Verify bone translation via device activation
- Standard surgical closure
The patient activation screwdriver mates with the activation hex on the distractor or an extension arm. The bone is advanced by the patient in 0.70 mm increments for one full rotation with the AB distractor and in 0.35 mm increments for one full rotation with the BC distractor, in accordance with the rate of distraction (number of turns per day) prescribed by the surgeon. To remove the distractor a second surgical procedure is necessary.
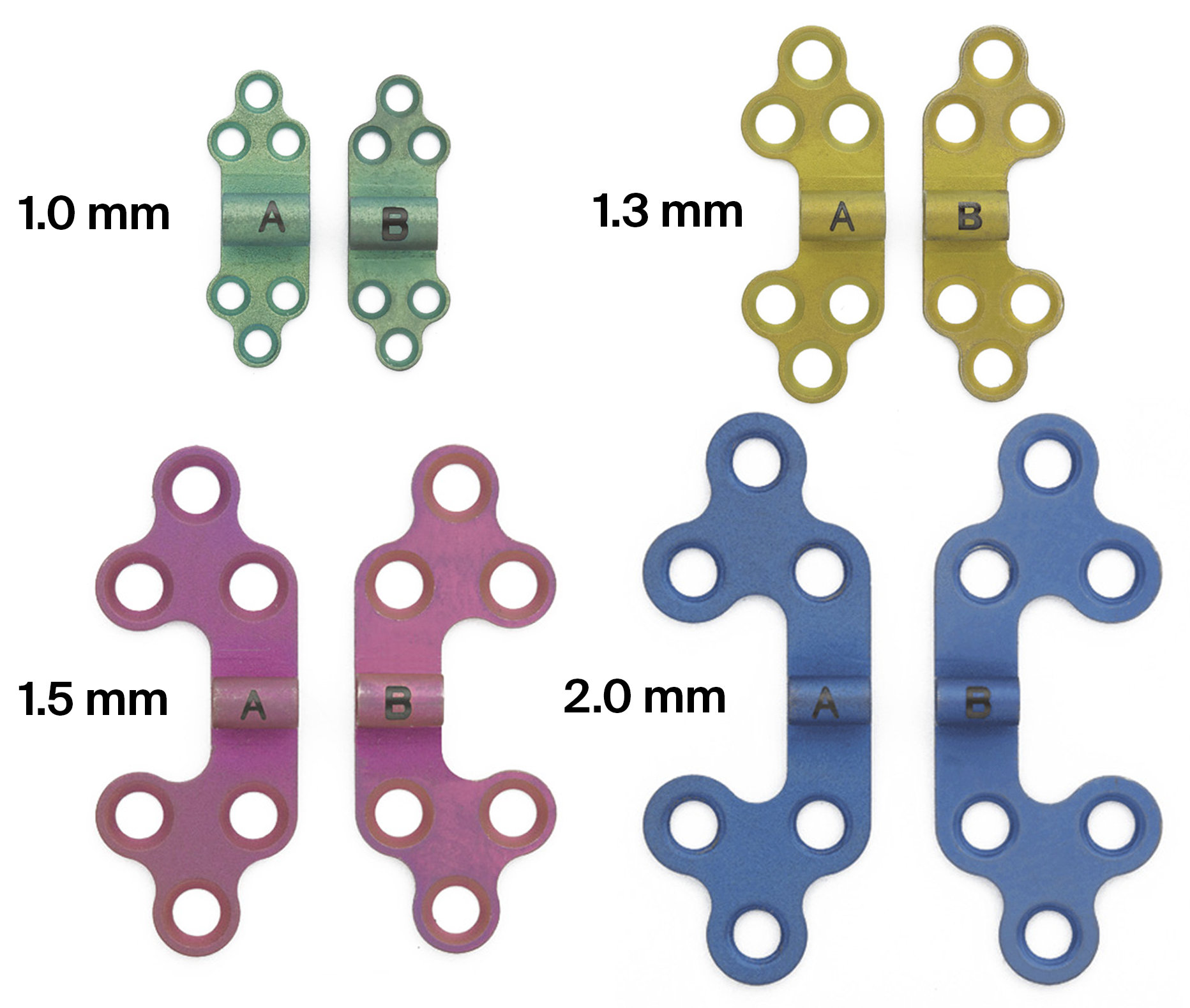
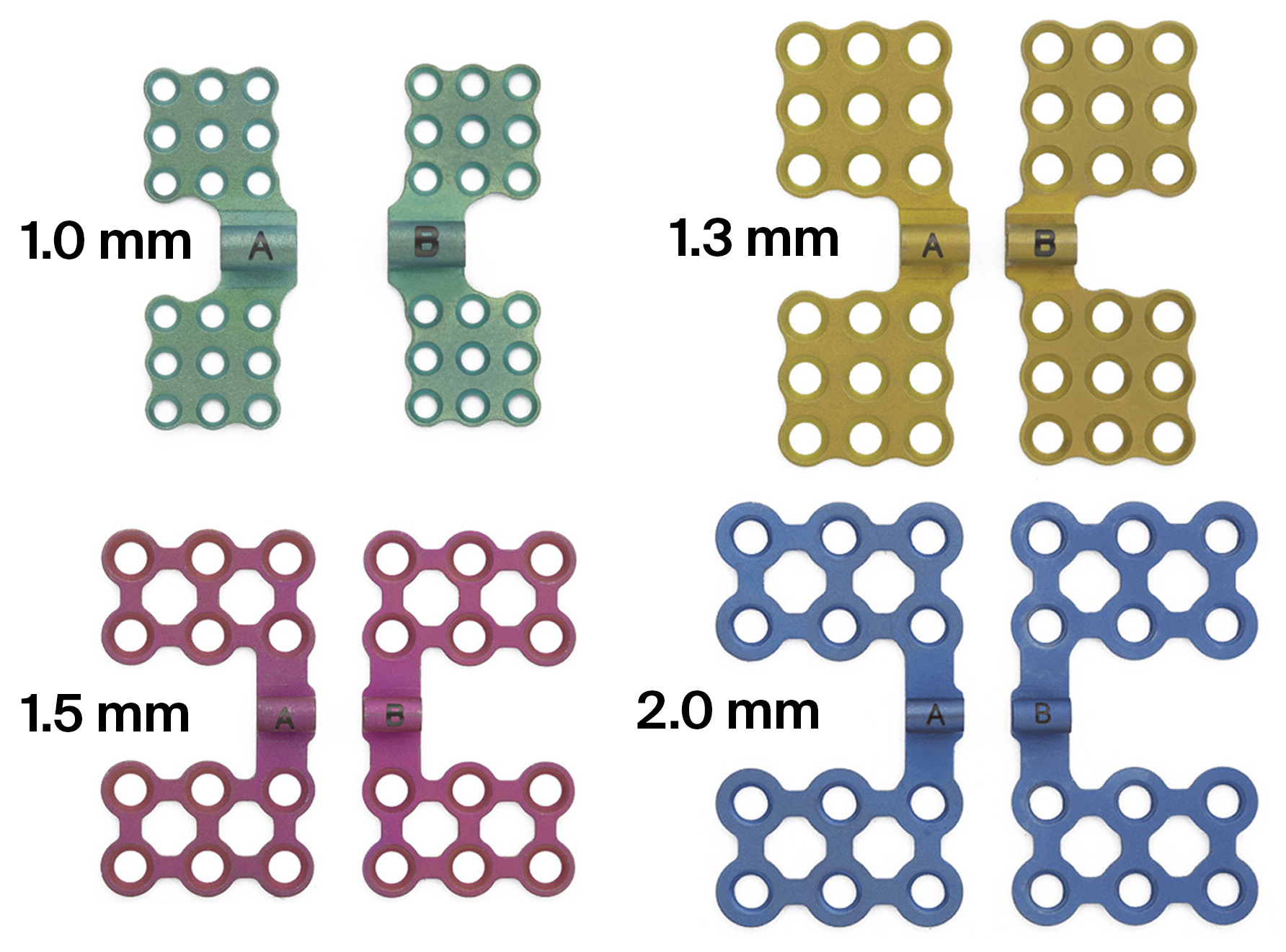
The CMF distraction system offers multiple styles and sizes of footplates that fit either with the AB or the BC distractor bodies. The footplates are suitable for use on both sides of the mandible and are designed with different screw-hole orientations to appropriately conform to individual patient anatomy. The footplates are available in 1.5 mm and 2.0 mm sizes and utilize 1.5 mm and 2.0 mm titanium bone screws respectively, to attach the device to the bone (Fig 6a-b).
To treat patients aged 12 months or younger, the new pediatric craniofacial distraction system is recommended. It has been developed using the same concept and comprises of 1.0 mm and 1.3 mm sized footplates which accept the corresponding bone screws. Both systems are intended for single-use only.
New K-wires for multi-vector distractor
Furthermore, new extensions to the existing multi-vector distractor system have been developed. Now, additionally available calibrated 2.0 mm K-wires can be used to secure the device to the mandible and transmit force to move the bone. They serve as a visual guide to control insertion depth.
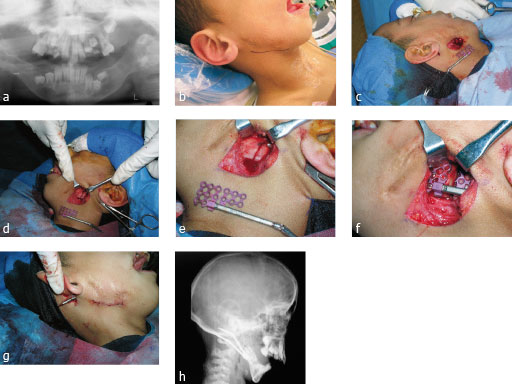
Distraction osteogenesis case
Case images courtesy of Scott P Bartlett, Philadelphia, US
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.