Distal Radius Intramedullary Nail (DRIM-Nail) for the fixation of extraarticular fractures
Ladislav Nagy
The Distal Radius Intramedullary Nail (DRIM-Nail) is an innovative treatment option indicated for unstable A3 and A2 extraarticular fractures of the distal radius. The design, development and certification of this new intramedullary nailing implant originally grew from a multi-partner collaboration between Disrad AG, a start-up founded at Balgrist University Hospital by the Balgrist Beteiligungs AG, the University of Zürich, the AO Technical Commission's Hand Expert Group, the AO's Innovation Funding, and 41medical. The DRIM-Nail addresses various clinical complications which may arise following fixation of distal radius fractures using state-of-the-art techniques such as closed reduction percutaneous pinning (CRPP) and open reduction and internal fixation (ORIF).
Distal radius fractures are the most common orthopedic injury accounting for 17.5% of all fractures in adults. These fractures generally occur from a fall on the outstretched hand, and roughly 50% of distal radius fractures are intraarticular. Diagnosis is made clinically and radiographically with orthogonal radiographs of the wrist. Treatment can be nonoperative or operative depending on fracture stability and fracture displacement as well as patients' age and activity demands.
Techniques for operative treatment include closed reduction percutaneous pinning (CRPP), open reduction and internal fixation (ORIF), and external fixation for fracture reduction and stabilization. While CRPP is an appropriate option for mainly extraarticular fractures, internal fixation using plates and screws remains the most common option for the surgical treatment of distal radius fractures. The primary goal of distal radius fracture fixation is to achieve and maintain anatomical reduction, thereby limiting any loss of function which is a significant outcome for young, active, and independent patients.
The implantation of plates and screws in the distal radius is appropriate for unstable, intraarticular, compound, and/or comminuted fractures and allows stable fixation compatible with early mobilization on only protective removable splinting. However, the main drawback of such internal fixation is the additional soft-tissue injury required for the exposure during implantation and thereafter the potential to cause tendon irritation near the implanted components, causing tendinopathy and possible tendon rupture. Currently, the most common complications directly related to distal radius plate fixation include tenosynovitis, tendon attrition, and rupture often necessitating hardware removal. The most obvious obstacle when adopting CRPP, besides the limited stability of the construct and the necessity of lengthy immobilization, is the extension of pins outside of the bone which can cause an increased risk of infection.
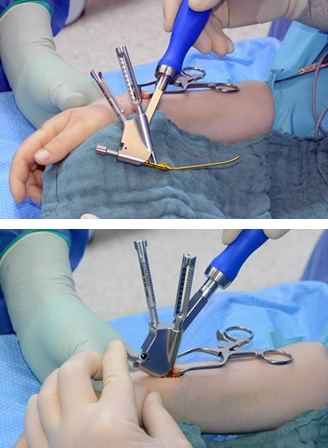
In response to such potential complications in the fixation of extraarticular distal radius fractures, the DRIM-Nail was designed and developed through a collaboration between Disrad AG, a start-up founded at Balgrist University Hospital by the Balgrist Beteiligungs AG, the University of Zürich, the AO Technical Commission's Hand Expert Group, the AO's Innovation Funding, and 41medical. The new device aims to decrease complications such as soft-tissue irritation. The implant utilizes the principles of load sharing, subchondral screw divergence, and locked fixed-angle fixation. The intramedullary implant is inserted through a small skin incision at the radial styloid and does not further devascularize the fracture fragments. The limited surgical dissection and rigid fracture fixation allows for early postoperative mobilization and an early return to function. The newly approved DRIM-Nail is a valuable addition to the arsenal of distal radius fracture treatment options, which can rapidly have patients on the path to recovery.
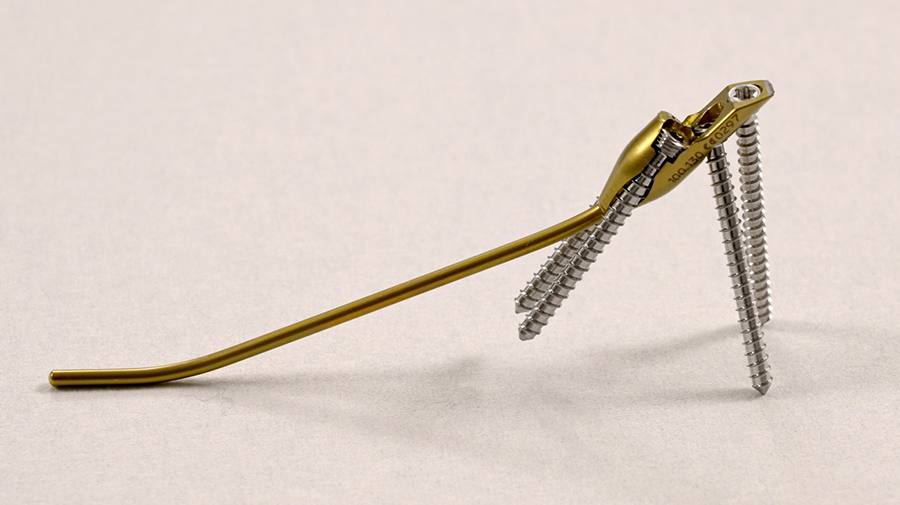
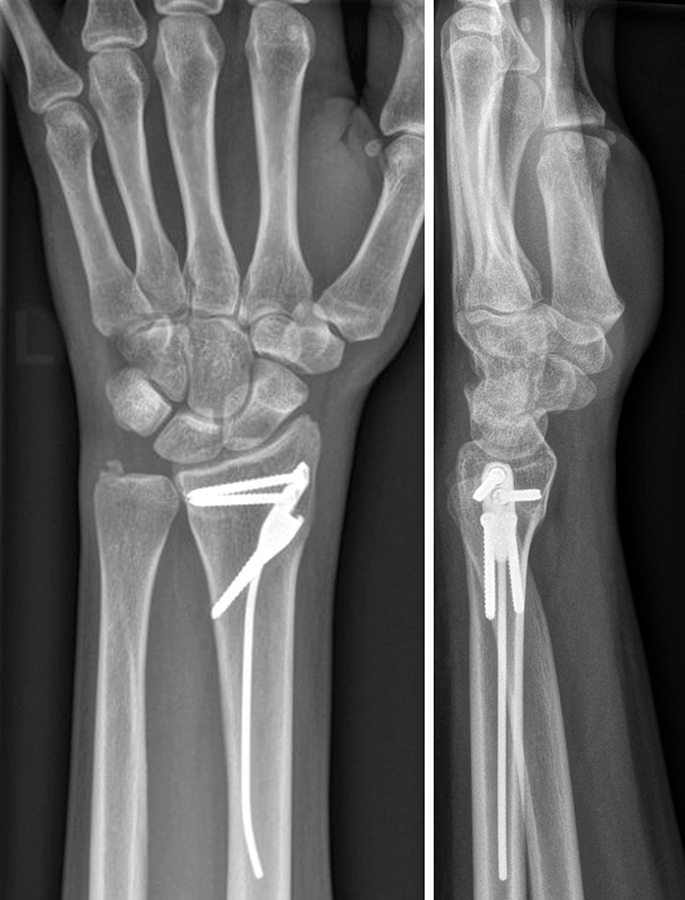
The DRIM-Nail is 7.0 mm in diameter and single packed as a 'one size fits all' sterile implant. The nail itself is made from titanium and the additional 2.5 mm diameter screws for fixation (ranging from 14−36 mm) are made of stainless steel (Fig 2). The instrument set is lean but comprehensive and intuitive to use. All instruments are nonsterile and validated according to 41medical reprocessing.
The development of the DRIM-Nail represents the eventual success of a long-standing AO Technical Commission project initiated in the Hand Expert Group in 2010 and driven specifically by Swiss surgeons Ladislav Nagy and Andreas Schweizer at Balgrist University Hospital in Zurich. After more than a decade in the making, the DRIM-Nail project has finally evolved from a concept to a market-ready product, marking a significant milestone in the history of the AO Technical Commission. Thanks to the successful collaboration with both Disrad AG and 41medical AG, the AO now has access to an intramedullary device for the fixation of extraarticular distal radius fractures.
Can your idea improve patient care or surgeon education?
Case 1
(Kindly provided by Ladislav Nagy, MD, Balgrist Universtity Hospital, Zurich, Switzerland)
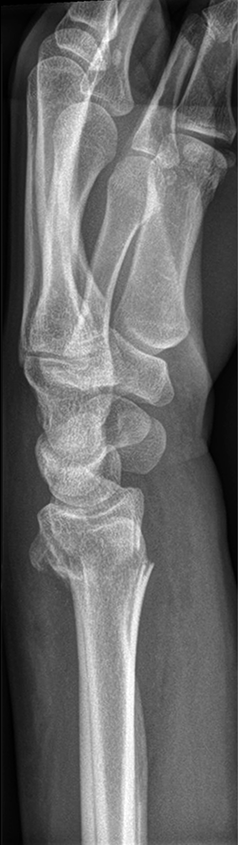
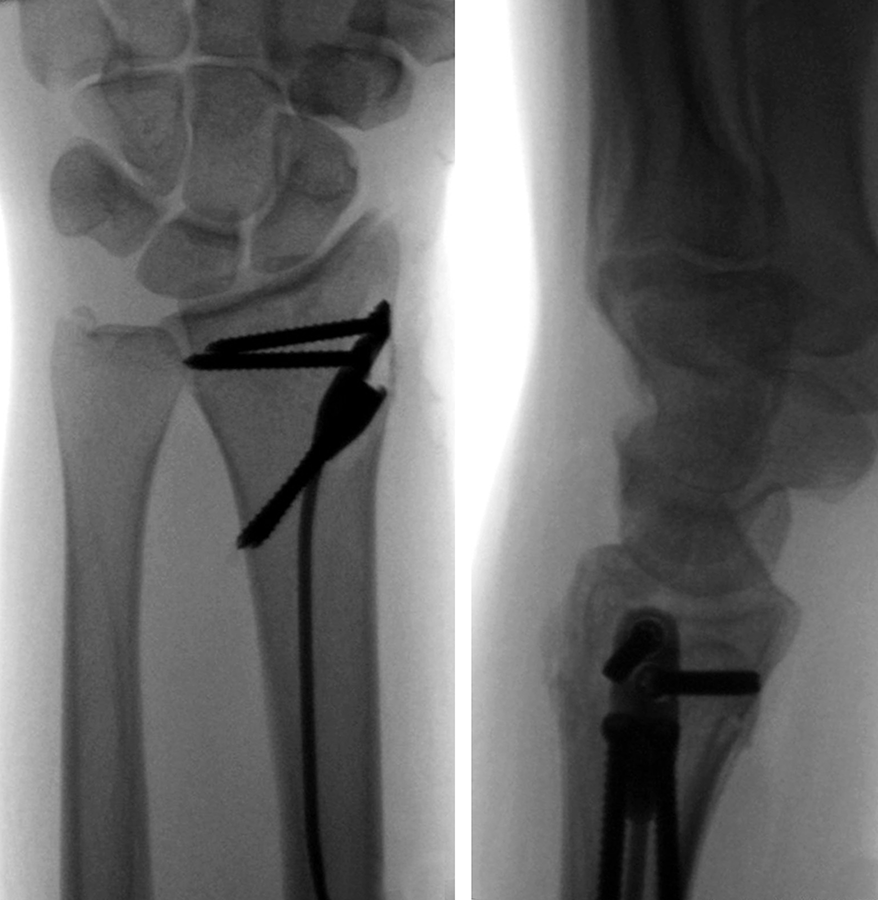
The patient was a 25-year-old woman who sustained a displaced, unstable extraarticular fracture of the left distal radius while snowboarding.
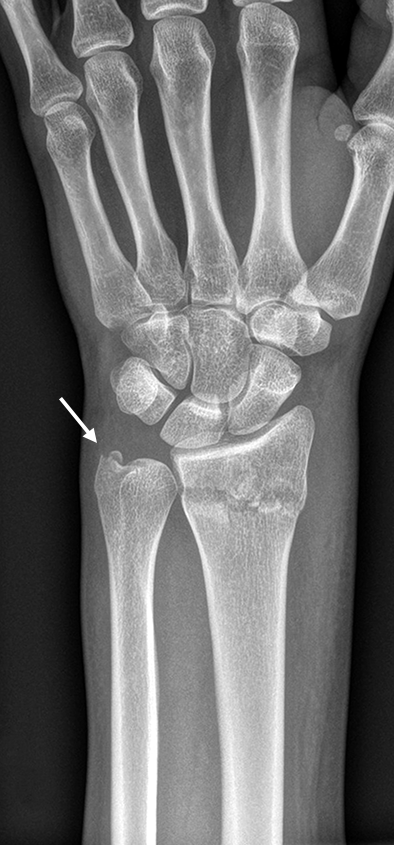
After closed reduction a dorsal re-dislocation of 25° occurred (Fig 3a). There are additional apparent signs of instability: dorsal comminution (Fig 3a) and fracture of the ulnar styloid (Fig 3b). Therefore, a considerable potential for further dislocation is present which is likely to occur in a cast. This young, active, and demanding patient wishes to return to her activities as early as possible. This can only be achieved with an operative fracture fixation. The nail was chosen instead of a plate. This allowed an almost instant functional use of her hand, which allowed her to return to work at 2 weeks postoperatively without additional fixation or a splint and full unprotected function of her wrist at 6 weeks.
Distal Radius Intramedullary Nail (DRIM-Nail) - 2024
This session may contain graphic medical procedures and imagery intended for educational purposes. Viewer discretion is advised. The content is designed for healthcare professionals and may include sensitive or disturbing material.
Treatment of Extraarticular Distal Radius Fractures with a Novel Intramedullary Nail - 2022
Watch the full video on World Surgery Tour TV here. All you need is to create a free account (no payment details are needed). You then find all videos (case introduction, surgery, and discussion) under "My Library" when scrolling down.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.