Modular Sternal Cable System
Please note that in 2011, this product has been superseded by the Sternal Zipfix System.
With a minimum of 345,000, in the US alone, annually performed surgical procedures that involve splitting the sternum to enter the thoracic cavity and gain access to the heart and other key organs, the need for effective sternal closure poststernotomy is vital. For the last few decades, surgeons have relied on stainless steel wires as their preferred chosen method of sternal closure. Although the reported failure rate of this technique is low (25%), many patients still experience pain, clicking, and other problems associated with nonunions that go untreated. The sternum is a unique bone located in an environment of constant motion. The thoracic cavity is highly vascularized, but a common practice of open-heart procedures is to remove one of the key blood supplies to the sternum for the heart. The bone is mostly cancellous with a very thin cortical shell, and its quality is often compromised, as many heart patients have other serious comorbidities such as:
- Chronic obstructive pulmonary disease (COPD)
- Obesity
- Diabetes
- Previous steroid therapy
- Osteoporosis
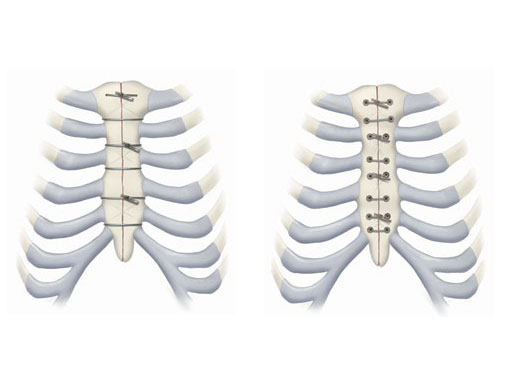
Problems with infection and failed closures have to be taken seriously, especially given the proximity to the heart. The need for an improved closure device has been recognized for many years and several products have attempted to solve this problem. For various reasons, none of these products have been able to obtain significant market share from the standard of care. The new Modular Sternal Cable System has been developed to address these needs. It consists of three basic implant components, which can be used alone or in combination, based on the surgeons preference and on patient condition.
Stainless Steel Cable
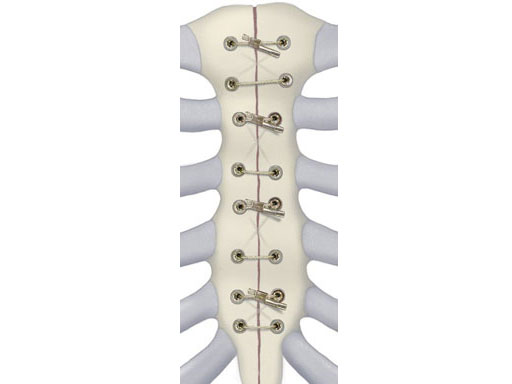
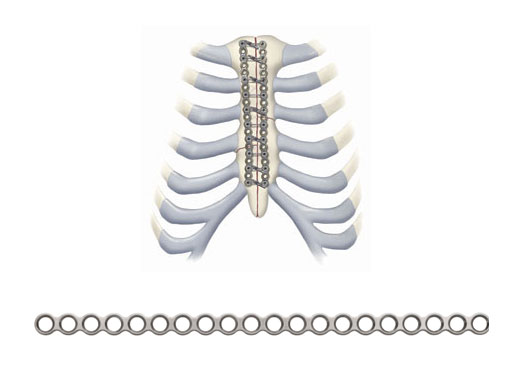
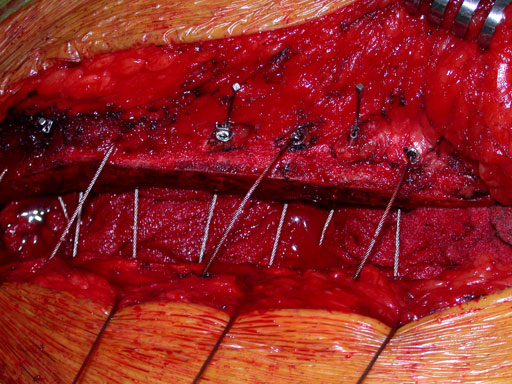
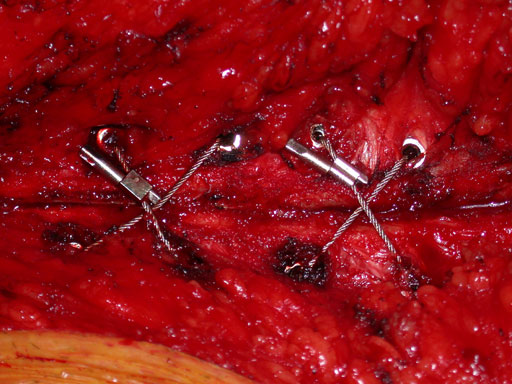
The principal component is a flexible stainless steel cable with a diameter of 1 x 1 mm. Contrary to standard wire, it has great strength, high flexibility, and a large surface area to allow for greater contact between bone and implant. Therefore the risk of pulling through bone is reduced. Each cable is supplied with a crimp fitting, which slides over the end of the cable and is crimped to maintain the tension in the cable. It can be used as a stand-alone solution for parasternal closure.
Cannulated Screw
For transsternal application of the cable, a blunt-tipped, self-tapping, stainless steel cannulated screw has been developed. The screw will be available in a variety of lengths at 1.0 mm increments for bicortical placement in the sternum with a minimum amount of protrusion through the posterior table. The screws serve as channels for the cable to pass through the sternum. This offers additional reinforcement instead of direct contact to the bone, therefore preventing it from damage. Reconstruction Plates Another component of the system is a series of stainless steel reconstruction plates. These plates are indicated for primary as well as secondary closures, for patients with limited sternum quality, and for secondary reconstruction of sternums that have fallen apart after initial surgery. The plates are fixed to the sternum with cannulated screws to reunite the hemi-sternums, which also serve as stress distributors. Depending on the surgeons preferred technique the cable is then applied through the cannulated screws and/or around the sternum and/or through the transverse holes in the plates to join and secure the two sternal halves.
Reconstruction Plates
Another component of the system is a series of stainless steel reconstruction plates. These plates are indicated for primary as well as secondary closures, for patients with limited sternum quality, and for secondary reconstruction of sternums that have fallen apart after initial surgery. The plates are fixed to the sternum with cannulated screws to reunite the hemi-sternums, which also serve as stress distributors. Depending on the surgeons preferred technique the cable is then applied through the cannulated screws and/or around the sternum and/or through the transverse holes in the plates to join and secure the two sternal halves.
Instruments
In addition to the implants in the set, a selection of instruments is offered, such as an instrument to tension, crimp, and cut the stainless steel cable. It is recommended to suture the cable directly through the sternum using the attached needle. Instrumentation to perforate the bone and facilitate cable passage when using cannulated screws is also available. Plate benders, plate cutters, depth gauges, screwdrivers and forceps are included in the system to facilitate installation of the implants. The screwdrivers have adjustable spade-bit tips that provide self-drilling functionality to the blunt-tip screws.
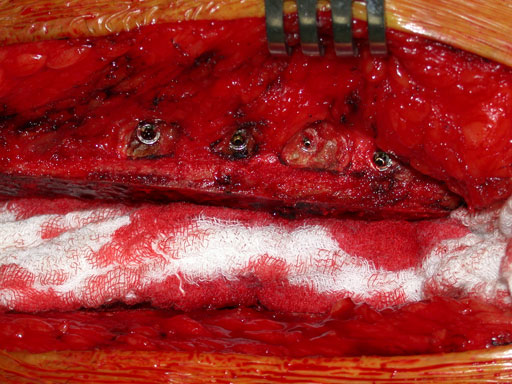
Closure of the sternum with cable.
Case images provided by Dr J Huh, Baylor College of Medicine, Texas Medical Center, USA.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.