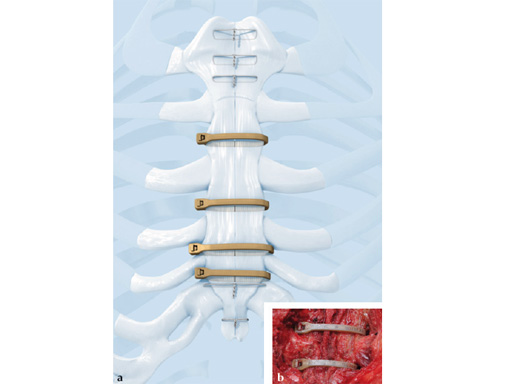
Sternal Zipfix System
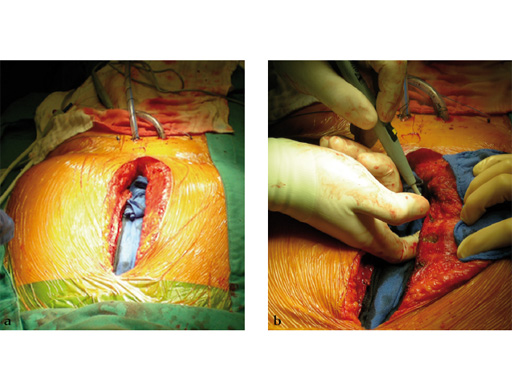
The sternal zipfix system primarily consists of polyetheretherketone (PEEK), biocompatible implants, which are similar to cable ties, and an application instrument. The purpose of this system is to achieve sternal closure following sternotomy by stabilizing the sternum and promoting fusion.
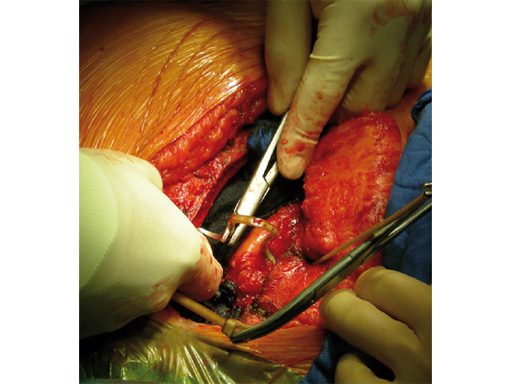
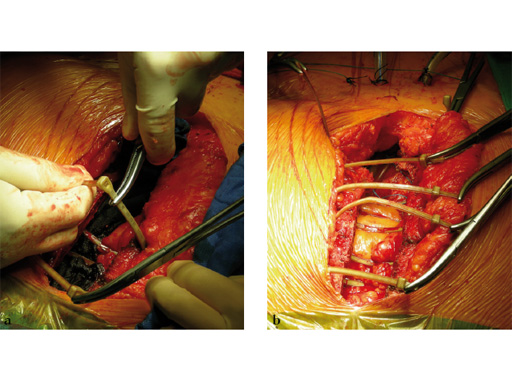
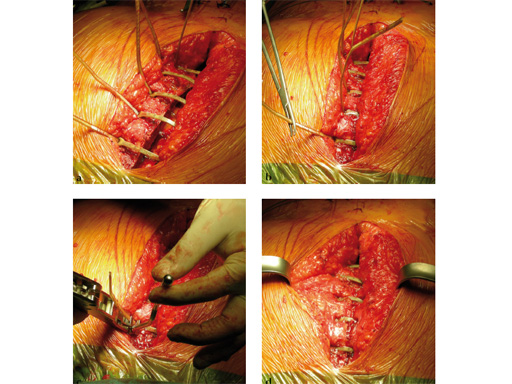
The implant itself comprises a removable stainless steel needle for peristernal application, the body with a ratchet mechanism, and a flat locking head. The application instrument is used to tension the implant, without overtensioning it, and also to cut it.
Case 1: A 66-year-old man with a history of chronic obstructive disease, obesity (BMI of 32.8), and transient ischemic attack was scheduled for a coronary artery bypass operation for stable angina.
The patients angiogram showed three-vessel coronary disease with good left ventricular function and an echocardiogram sinus rhythm.
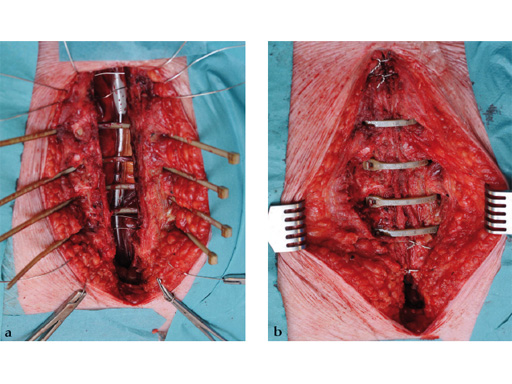
The hybrid sternal closure technique with stainless steel wires and sternal Zipfix has been used in the authors' institution in 50 patients without any instance of sternal instability or dehiscence.
Case provided by Ted Elenbaas and Sander Wolters, Eindhoven, The Netherlands
Case 2: A 55-year-old woman, who previously underwent ventricular septal defect closure at 5 and aortic valve reconstruction at 31, required aortic valve replacement due to symptomatic aortic valve insufficiency.
The patient was morbidly obese with a BMI of 45. Due to the high risk for sternal instability and/or deep sternal wound infection, closure was performed using the sternal zipfix system. The zipfix provides quick and reliable stable fixation of the sternum even in patients that are at a higher risk to develop a sternal instability or a deep sternal wound infection.
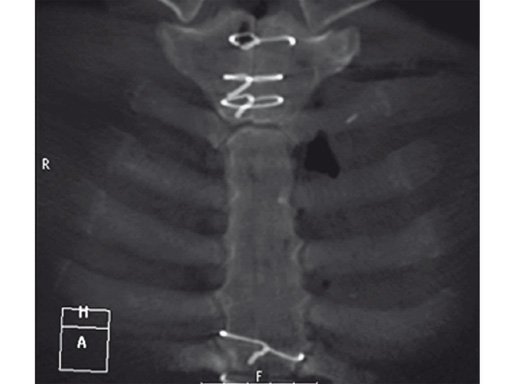
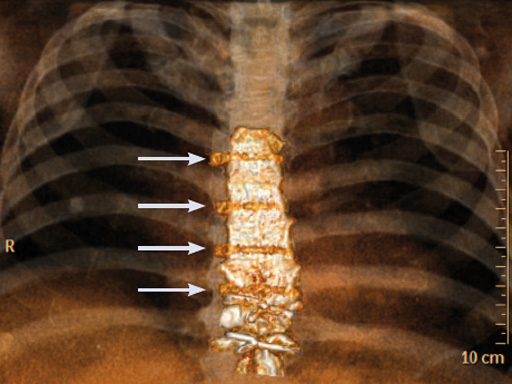
The postoperative course was uneventful and the patient was discharged on postoperative day 7 with no signs of sternal instability or wound healing problems. At 18 months follow-up the wound had healed completely and the sternum was stable, with no signs of infection.
Case provided by Roman Gottardi, Salzburg, Austria
Median Sternotomy Closure
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.