VOLT™ Wrist Treatment System: Two-Column Distal Radius Plates
Tom Fischer, Sudhir Warrier, Alex Lluch, Martin Langer, Stephen Tham
The VOLT™ (Variable Angle Optimized Locking Technology) Wrist Treatment System is a comprehensive plating solution designed to address the entire spectrum of wrist fractures. Comprising standard volar and dorsal fixation for basic fractures, specialized plating options for more complex fractures, dedicated implants for elective procedures, and wrist-specialized instrumentation, the plating system allows fixation of very distal wrist fractures without invading articular space or causing post-operative soft tissue concerns.
VOLT™ technology allows for the design of low-profile plates without compromising stability or locking strength. The enhanced design of the new system facilitates the entire surgical procedure, aiding fracture reduction, plate placement, and screw insertion. All instruments within the VOLT™ Wrist Treatment System have been re-designed to provide surgeons the same user experience across all VOLT™ systems, and ultimately to allow the streamlining of inventory.
The VOLT™ Two-Column Distal Radius Plates - standard (Fig 1) and rim (Fig 2) versions -build on the heritage of the well-researched LCP and VA-LCP predecessors, with enhanced design features that allow Variable Angle Optimized Locking Technology (VOLT™) to be combined with pre-contoured plates refined through extensive anatomic research. The plates address the clinical need for fixation of small and very distal bone fragments, while keeping the plate profile distanced from the path of the flexor pollicis longus (FPL) tendon.
VOLT™ Volar and Dorsal Distal Radius Plates were approved by the AO Technical Commission in September 2025 as a complementary extension to the VOLT™ Wrist Treatment System.
Design features of VOLT™ Two-Column Distal Radius Plates
The VOLT™ Two-Column Distal Radius Plates are low profile and feature rounded edges in the proximity of the flexor pollicis longus (FPL) tendon. Markings are incorporated in the shaft region to help with plate adjustment (Fig 2), and in the distal plate to indicate nominal screw direction. The plates fit a wide range of radius anatomies and offer more distal placement on the ulnar side and better targeting of the radial styloid than their predecessors.
The VOLT™ screw holes are threaded and accept either 2.4 mm or 2.7 mm cortex or locking screws in any hole. VOLT™ screw holes have a 30-degree cone of angulation with locking screws (Fig 3). The radial screw holes are more steeply angled to direct the screw towards the styloid tip, allowing the capture of distal bone fragments (Fig 2). The additional VOLT™ Two-Column Distal Radius Rim plate version (Fig 2) features low profile ulnar and radial extensions to help control distal fragments while avoiding the region of the FPL tendon.
The system includes radiolucent guide blocks that feature safe screw angles directing implants and instruments away from the wrist joint for sequential drilling and screw insertion. Built-in 1.6mm K-wire holes indicate the approximate trajectory of the adjacent distal ulnar and radial screws to assist with plate placement using reference K-wires (Fig 2). The system is fully compatible with the VOLT™ Mini Fragment Plating System, and its modular design allows the system to be either self-contained or used as an add-on to the existing system.
Indications
The VOLT™ Wrist Treatment System includes distal radius, forearm, and fragment-specific plates, which are indicated for fixation of fractures, fusions, non-unions and malunions, or osteotomies of the radius, ulna, and hand.
The VOLT™ Wrist Treatment System is not for spinal use.
Contraindications
- Infection.
- Patient conditions including blood supply limitations, obesity and insufficient quantity or quality of bone.
- Patients with mental or neurologic conditions who are unwilling or incapable of following postoperative care instructions.
- Foreign body sensitivity. If material sensitivity is suspected, testing is required prior to implanting the device.
Clinical case kindly provided by Amy Speeckaert, Department of Orthopaedic Surgery, Ohio State University
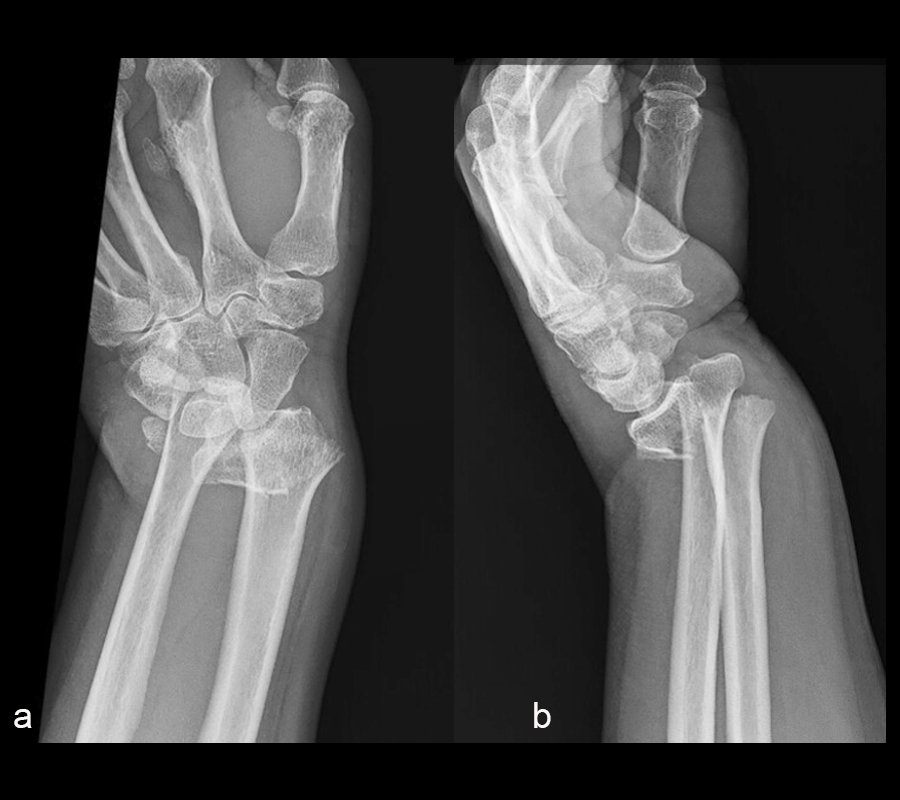
A 60-year-old woman fell on ice from standing height and sustained an injury to the right wrist. Upon admission to the hospital, x-rays revealed a comminuted, intra-articular distal radius fracture with significant dorsal translation and angulation (Fig 1). The patient’s previous medical history included:
- Type 2 diabetes treated with oral medication
- Obesity
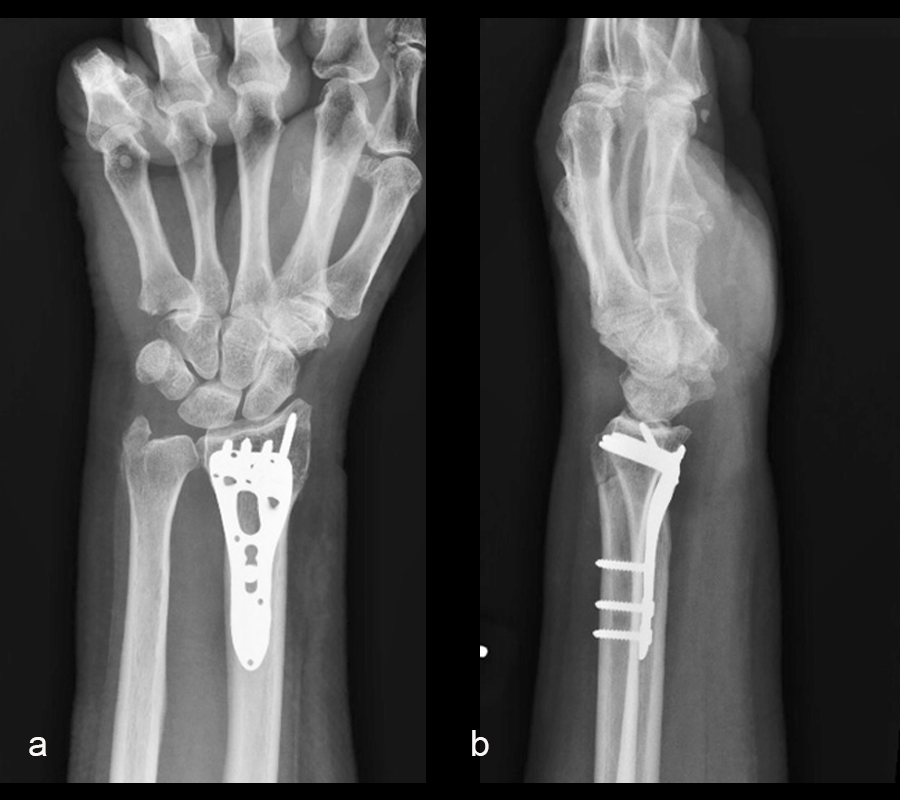
The distal radius fracture was treated with open reduction and internal fixation through a volar Henry Approach, using a VOLT™ Two-Column Distal Radius plate. At the 6-week follow-up, x-rays demonstrated a healed fracture (Fig 2). The patient’s pain had resolved and she was regaining range of motion with occupational therapy.
VOLT™ Wrist Treatment System
This session may contain graphic medical procedures and imagery intended for educational purposes. Viewer discretion is advised. The content is designed for healthcare professionals and may include sensitive or disturbing material.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.