TriALTIS™ Spine System
Christian Mazel, Naresh Kumar, Firoz Miyanji, Yu-Mi Ryang, Kevin Seex
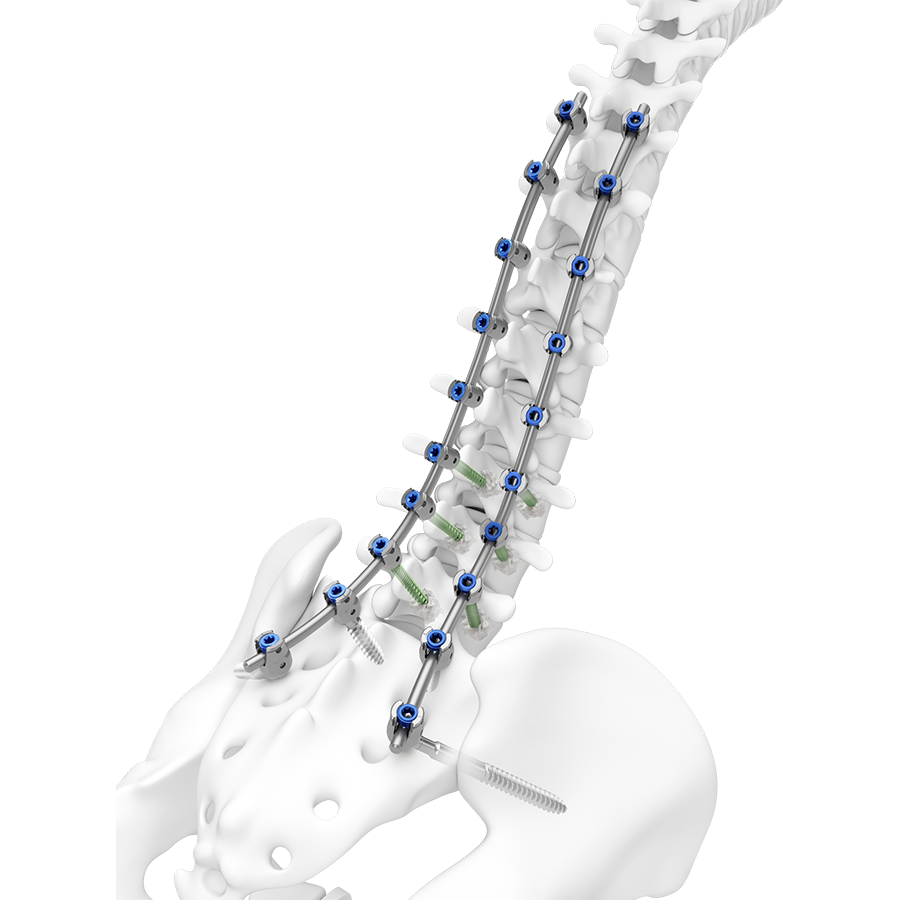
The TriALTIS™ Spine System is a next generation posterior thoracolumbar spine implant system with a clinical focus on open surgery for degenerative, tumor, trauma and deformity pathologies in adults. Combining a new portfolio of implants with digital enabling technology, the TriALTIS™ Spine System aims to address unmet clinical needs and help surgeons achieve more consistent outcomes in treating complex spine conditions. Novel implants include polyaxial screws, favored angle screws, set screws, rods, and instruments.
Leveraging design features of the EXPEDIUM™ 5.5, VIPER™ Spine, and MATRIX™ Spine Systems, the TriALTIS™ Spine System delivers a consistent user experience and improved implant performance in terms of set screw clamping force, set screw loosening torque, resistance to pullout, and gripping capacity. Other innovations include improved instrument design to indicate actuation points, consolidated instrument sets, and a modular case and tray design.
The TriALTIS™ system affords efficient integration with cement augmentation, power, navigation and robotic-assisted solutions, while decreasing costs to healthcare systems and addressing the needs of more patients. The TriALTIS™ Spine System is the foundational element of a wider comprehensive system (coming soon) that will allow for a consistent surgeon user experience across all indicated pathologies.
Clinical cases kindly provided by Firoz Miyanji, MD, Department of Orthopedics, University of British Columbia, Canada
Case 1
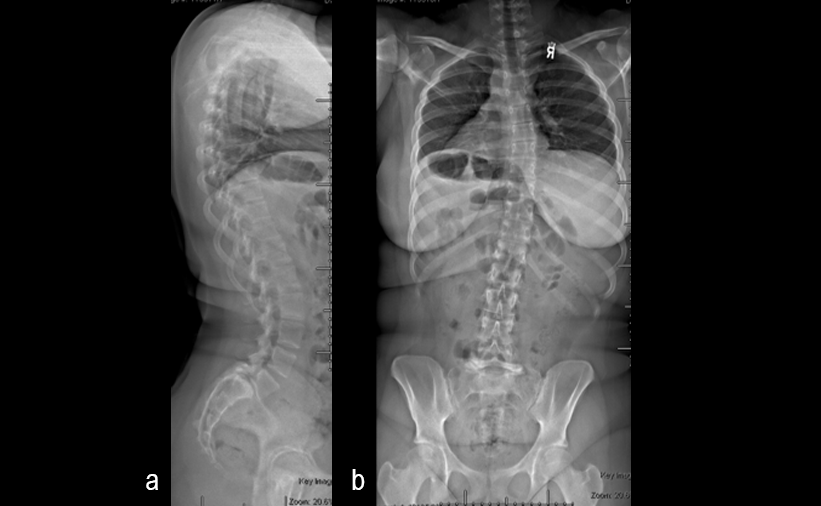
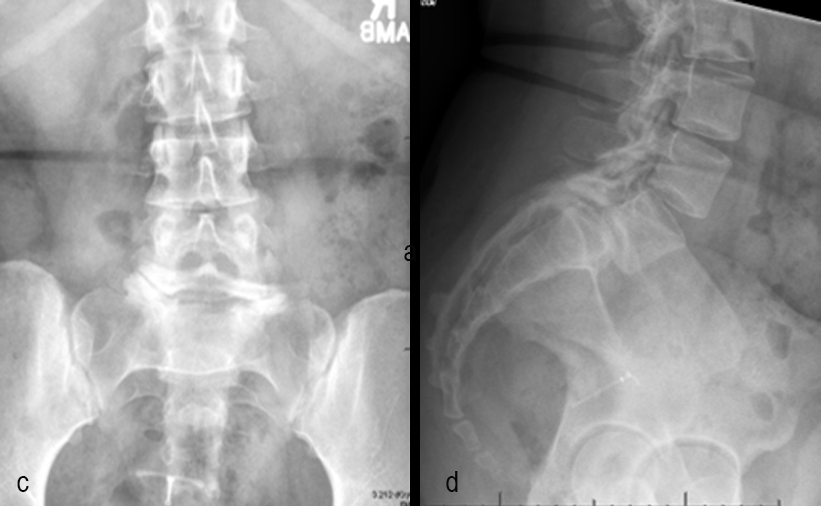
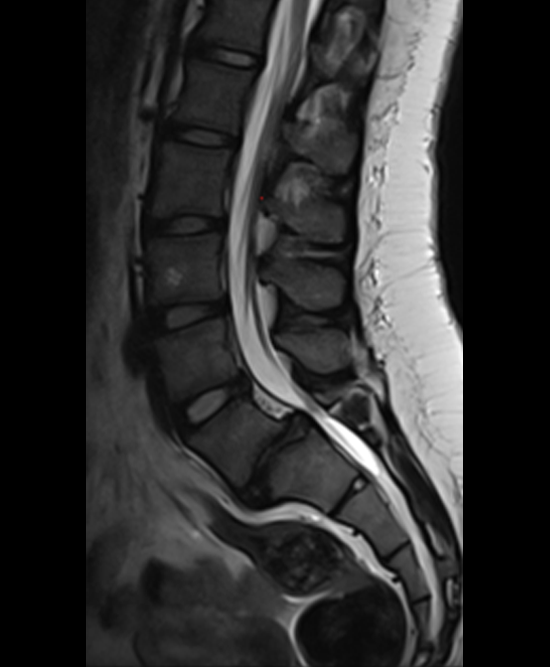
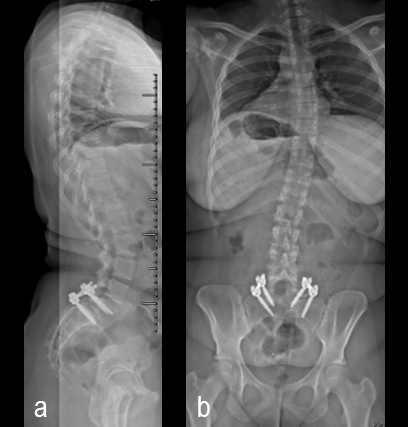
A 17-year-old girl presented to the spine clinic with significant low back pain. X-ray imaging (Figs 2-4) showed evidence of spondylolisthesis. Upon clinical examination of spinal alignment, the patient was found to have a pelvic incidence of 78°, a lumbar lordosis of 54°, and a pelvic tilt of 51°.
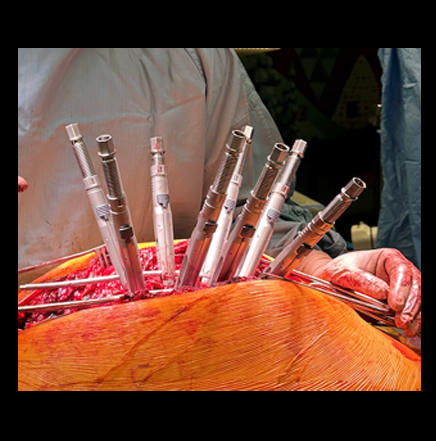
Posterior spinal instrumentation and fusion (PSIF) was undertaken with an interbody fusion at L5-S1. L5 laminectomy and sacral dome osteotomy followed by pedicle screw instrumentation using TriAltis™ implants helped reduce the lumbosacral kyphosis and translation of L5-S1 and promote fusion across this level (Fig 5a-b).
Case 2
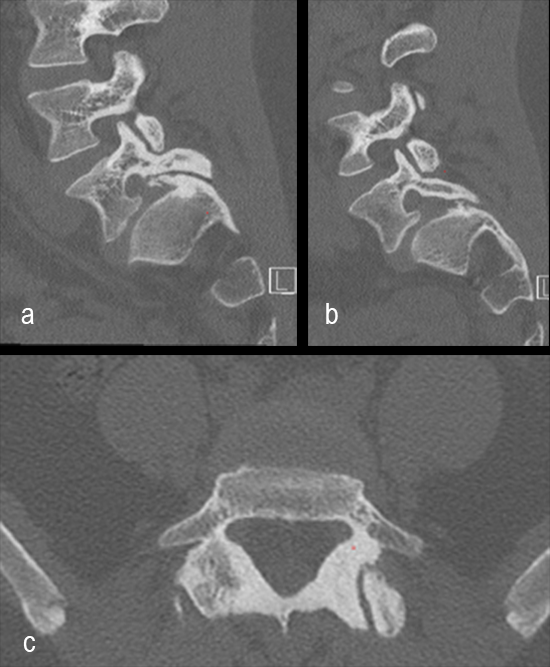
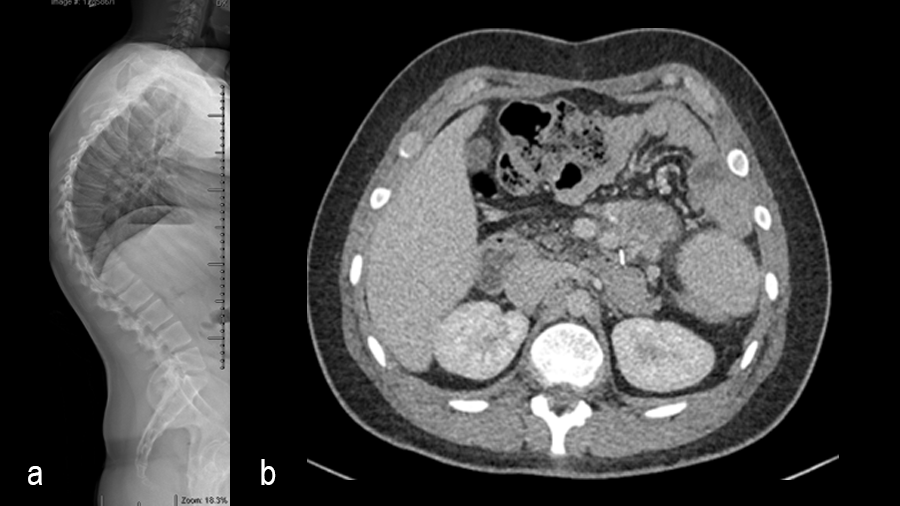
A 19-year-old man with colon cancer was admitted to the spine clinic with pain and progressive kyphosis (Fig 6a). Preoperative CT imaging (Fig 6b) showed a residual mass abutting the superior mesenteric artery.
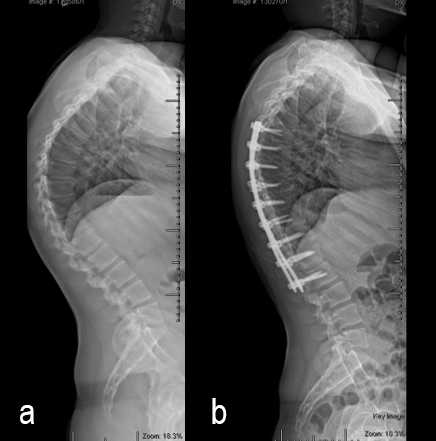
Surgery was undertaken with TriAltis™ posterior spinal instrumentation system from T5 – L1 to correct and support the kyphotic deformity (Fig 7a-b). The upper and lower instrumented vertebrae were selected to allow symmetry of fixation above and below the apex of the kyphotic curve. Care was taken not to overcorrect the hyperkyphosis given the abdominal mass abutting the superior mesenteric artery. During surgery, multiple sequential reducers were used to achieve cantilever reduction of the deformity (Fig 8).
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.