MatrixSTERNUM™ Fixation System
Mario Gasparri, Stefan Schulz-Drost, Mike Bemelman, Peter Cole, Ledford Powell, Evert Eriksson
The innovative MatrixSTERNUM™ Fixation System provides a low-profile plating solution for sternotomy and thoracotomy repair that is based on the locked plating technology offered in the MatrixRIB Plating System.
Containing a variety of 1.5 mm titanium sternal body and thoracotomy locking plates, screws, screw guides, instruments, modules, and trays, the MatrixSTERNUM™ Fixation System addresses the critical need for faster and more precise plate fixation following cardiac surgery, particularly for high-risk patients. The system includes dedicated multi-hole screw guides that reduce the number of steps and intraoperative time required for plate placement and screw insertion by the surgeon.
The MatrixSTERNUM™ Fixation System is designed for strength, operating room efficiency, and ease of use. The system is proven to provide three times greater construct locking strength compared to current products on the market.
In addition, multi-hole Screw Guides result in 43% faster screw insertion compared to current products on the market. The low-profile plates in the system are 1.5 mm thick for contourability and low palpability.
Clinical needs addressed
Every year in the U.S., approximately 200,000 people undergo coronary artery bypass graft (CABG) surgery, making it the most common type of heart surgery [1]. Ensuring chest-wall stability post-surgery is critical for patient recovery.
Surgeons need a fast, simple, and easy-to-use solution for rigid sternal fixation that minimizes primary closure time after an often-lengthy cardiothoracic procedure. Additionally, plate thickness is a significant concern as surgeons wish to simplify plate contouring and reduce the palpability of the implants.
Current sternal fixation systems often lack sufficient locking strength and are time-consuming to implant. Furthermore, they offer thicker plates that can be noticeable under the skin and uncomfortable for patients. Innovations in sternal fixation techniques are urgently needed to promote better healing, reduce complications, and reduce recovery times.
The MatrixSTERNUM™ Fixation System addresses these clinical needs by offering enhanced locking strength, faster and more precise fixation, and low-profile, highly contourable plates. This advanced system addresses the specific needs of patients, surgeons, and healthcare providers, and aims to provide better outcomes and improved patient comfort.
Product details
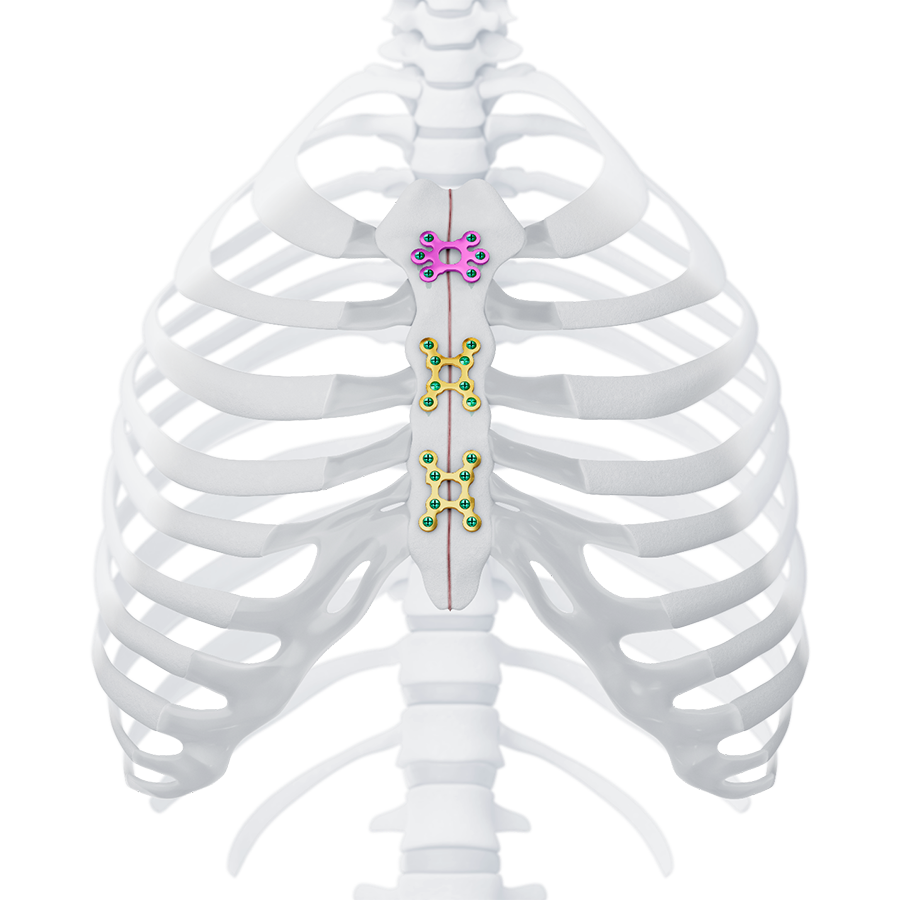
The MatrixSTERNUM™ Fixation System (Fig 3) consists of two families of plates and screws:
1. MatrixSTERNUM™ Sternal Body Plates
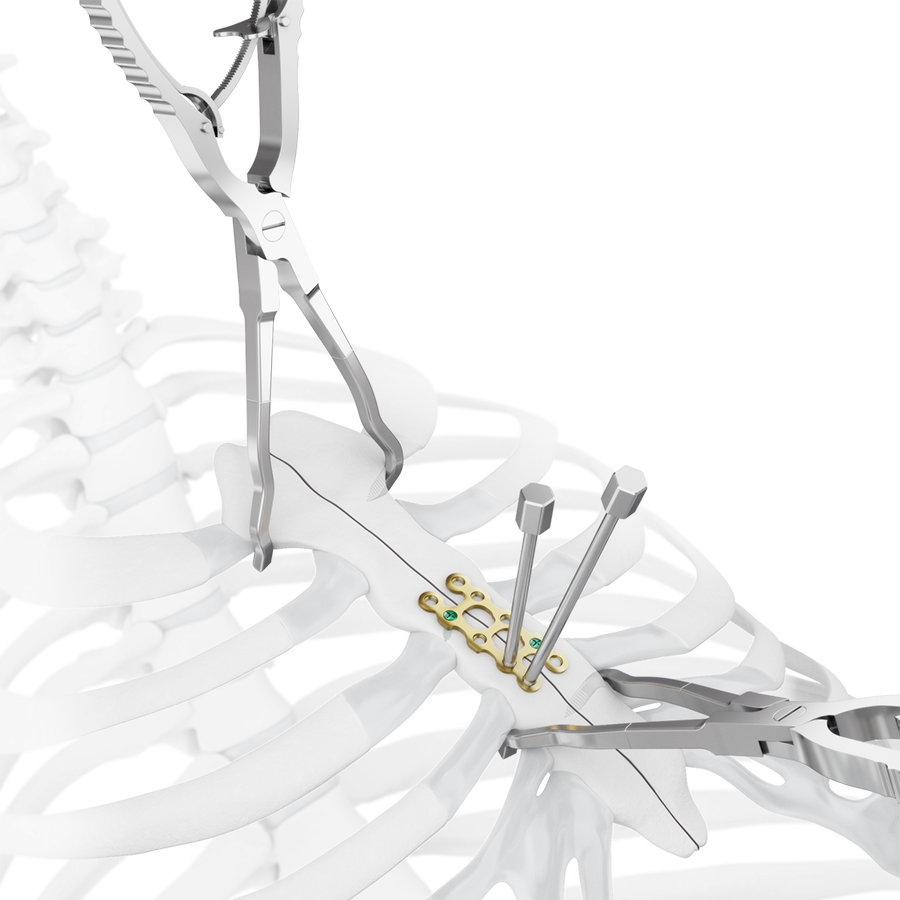
Indicated for internal fixation of bone discontinuities following sternotomy (Fig 3 and Fig 7).
- 12 plate shapes/sizes, color-coded for size (width)
- Material: CP Ti Grade 4
- Plate thickness: 1.54–1.60 mm
2. MatrixSTERNUM™ Thoracotomy Plates
Indicated for internal fixation of bone and/or cartilage discontinuities following thoracotomy (Fig 3).
- Three plate shapes/sizes
- Material: TAN
- Plate thickness: 1.54 mm
The plates are available in different shapes and sizes and are made from titanium alloy or commercially pure titanium. The sternal body and thoracotomy plates are designed for emergent re-entry.
MatrixSTERNUM™ Self-Drilling Locking and Non-Locking Screws
Designed for stabilization and fixation of bone in the anterior chest wall (Fig 3 and Fig 4).
- Locking Screws: Ø2.7 mm, self-drilling, 8–20 mm long, 1 mm increments
- Non-Locking Screws: Ø2.7 mm, self-drilling, 10 mm and 12 mm long
- Material: TAN
The system’s locking screws are offered in 1 mm-increment lengths for better bicortical fit and fixation, with lengths ranging from 8–20 mm. The implants are color-coded, simplifying the plate fixation procedure for the surgeon.
Indications for the MatrixSTERNUM™ Fixation System
The MatrixSTERNUM™ Fixation System is indicated for use in adults with normal bone quality.
- MatrixSTERNUM™ Sternal Body Plates are indicated for internal fixation of bone discontinuities following sternotomy.
- MatrixSTERNUM™ Thoracotomy Plates are indicated for internal fixation of bone and/or cartilage discontinuities following thoracotomy.
Contraindications
- MatrixSTERNUM™ Thoracotomy Plates are contraindicated for screw attachment or fixation to the clavicle or spine.
Key features and benefits
The MatrixSTERNUM™ Fixation System offers several key benefits:
- Enhanced locking strength: The system provides three times greater construct locking strength compared to the market-leading product, ensuring better fixation of the sternum during the critical postoperative healing period.
- Speed and efficiency: The multi-hole Screw Guides allow for rapid and precise plate fixation, significantly reducing the surgical procedure time via a reduced number of steps for plate placement and screw insertion. Overall, screw insertion is 43% faster compared to the market-leading product.
- Low-profile design: The 1.5 mm low-profile plates are thinner, making them less palpable under the skin and enhancing patient comfort.
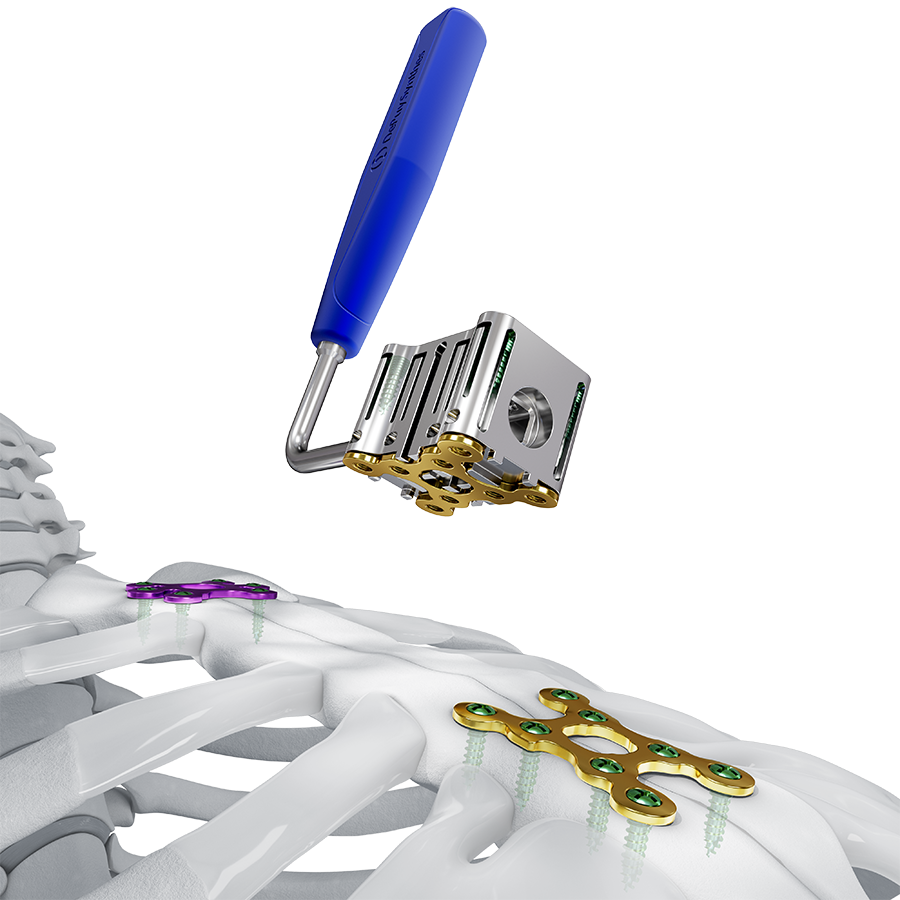
- Contourability: The plates are highly contourable, allowing them to be adapted to the sternal and rib anatomies. In-situ Bending Instruments, Combination Bending Pliers, and Bending Pliers are offered with the system.
- Comprehensive plate offerings: The system features a wide range of plate options. This reduces the need for plate cutting to adapt plates to the sternal and rib anatomies.
Clinical impact
Recent research indicates that compared to wire cerclage, using rigid plates for fixation following sternotomy can help to reduce sternal complications for high-risk patients, reduce perioperative mortality rates, and shorten hospital stays [2].
Wire cerclage is a long-established and low-cost solution for sternal closure that offers adequate outcomes [3]. However, the ability of wire cerclage to provide rigid fixation and prevent sternal movement is limited [3]. The resulting sternal instability can be associated with complications including bony nonunion, infection, and dehiscence, especially in high-risk patient populations [4]. In contrast, rigid fixation of the sternum may prevent sternal instability and complications, particularly in high-risk patients, compared to conventional wires and cables [2, 5].
A recent randomized trial showed that compared to wire cerclage, patient-reported outcome measures were better in rigid plate fixation, including reduced sternal pain, better sternal healing and quality-of-life scores, and improved upper extremity function. Furthermore, economic outcomes (total costs) were similar between wire cerclage and rigid plate fixation at 90 days post-surgery [3].
A 2018 prospective study found that when compared to wire cerclage, rigid plate fixation resulted in better sternal healing scores and higher sternal union rates at 3- and 6-months post-surgery, and fewer sternal complications at 6 months post-surgery [6]. However, a 2019 study highlighted the surgical challenges inherent to high-risk patients, indicating that despite the use of rigid plate fixation, smokers remained at increased risk for surgical site infection and sternal dehiscence [4].
The 2019 consensus recommendations for sternal closure published by Enhanced Recovery After Surgery (ERAS) advise that rigid sternal fixation has benefits in patients undergoing sternotomy and can be useful to improve or accelerate sternal healing and reduce mediastinal wound complications. Furthermore, the guidelines advise that rigid sternal fixation should be especially considered in individuals at high risk (eg, patients with a high body mass index, previous chest-wall radiation, severe chronic obstructive pulmonary disorder, or steroid use) [7].
In line with the advantages of rigid plate fixation over wire cerclage noted above, the MatrixSTERNUM™ Fixation System provides stronger, faster, and thinner solutions for rigid plate fixation that streamline sternotomy and thoracotomy procedures and offer surgeons greater flexibility to meet patient needs.
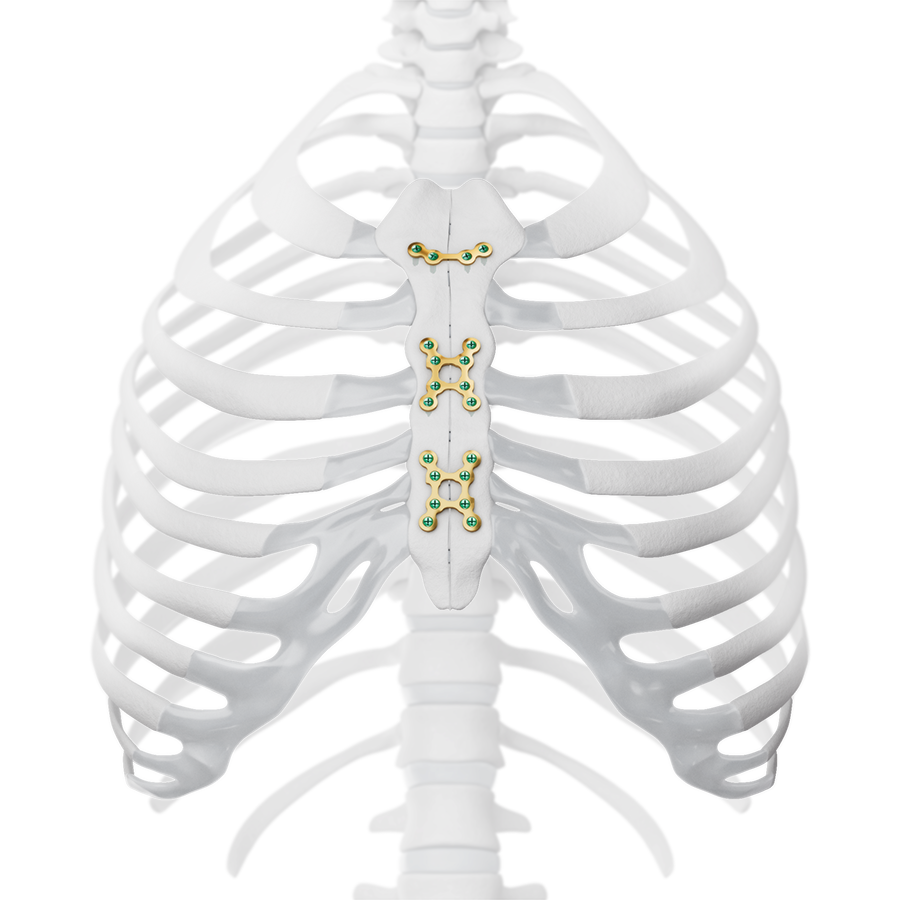
The development of the MatrixSTERNUM™ Fixation System (Fig 7) involved close collaboration with expert surgeons from the AO Technical Commission. This ensured direct input from leading professionals in the field, addressing real-world clinical challenges and optimizing the system for practical use in surgical settings.
References
- Jan A, Hayat MK, Khan MAA, et al. Trends in per-operative parameters and postoperative complications associated with coronary artery bypass graft surgery (CABG); A four-year retrospective study. Pak J Med Sci. 2021 Nov-Dec;37(7):1734–1739.
- Tam DY, Nedadur R, Yu M, et al. Rigid Plate Fixation Versus Wire Cerclage for Sternotomy After Cardiac Surgery: A Meta-Analysis. Ann Thorac Surg. 2018 Jul;106(1):298–304.
- Allen KB, Thourani VH, Naka Y, et al. Rigid Plate Fixation Versus Wire Cerclage: Patient-Reported and Economic Outcomes From a Randomized Trial. Ann Thorac Surg. 2018 May;105(5):1344–1350.
- Tran BNN, Chen AD, Granoff MD, et al. Surgical outcomes of sternal rigid plate fixation from 2005 to 2016 using the American College of Surgeons-National Surgical Quality Improvement Program database. Arch Plast Surg. 2019;46(4):336–343.
- Fawzy H, Alhodaib N, Mazer CD, et al. Sternal plating for primary and secondary sternal closure; can it improve sternal stability? J Cardiothorac Surg. 2009 May 7:4:19.
- Allen KB, Icke KJ, Thourani VH, et al. Sternotomy closure using rigid plate fixation: a paradigm shift from wire cerclage. Ann Cardiothorac Surg. 2018 Sep;7(5):611–620.
- Engelman DT, Ben Ali W, Williams JB, et al. Guidelines for Perioperative Care in Cardiac Surgery: Enhanced Recovery After Surgery Society Recommendations. JAMA Surg. 2019 Aug;154(8):755–766.
MatrixSTERNUM™ Instruments
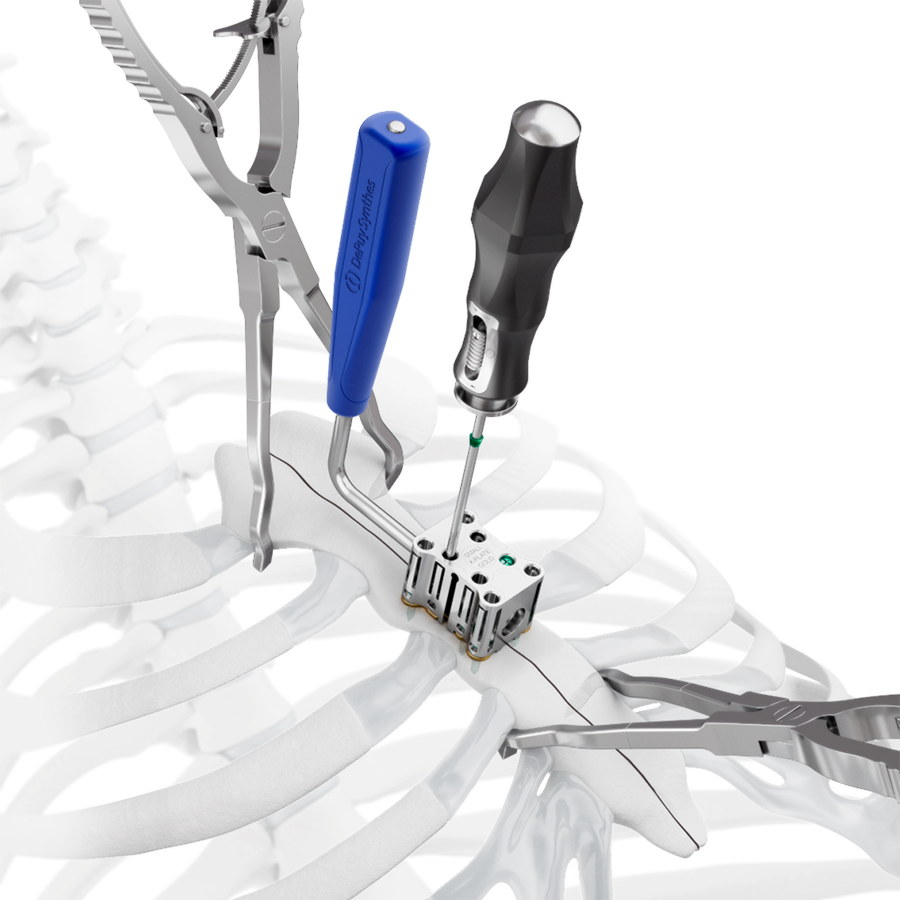
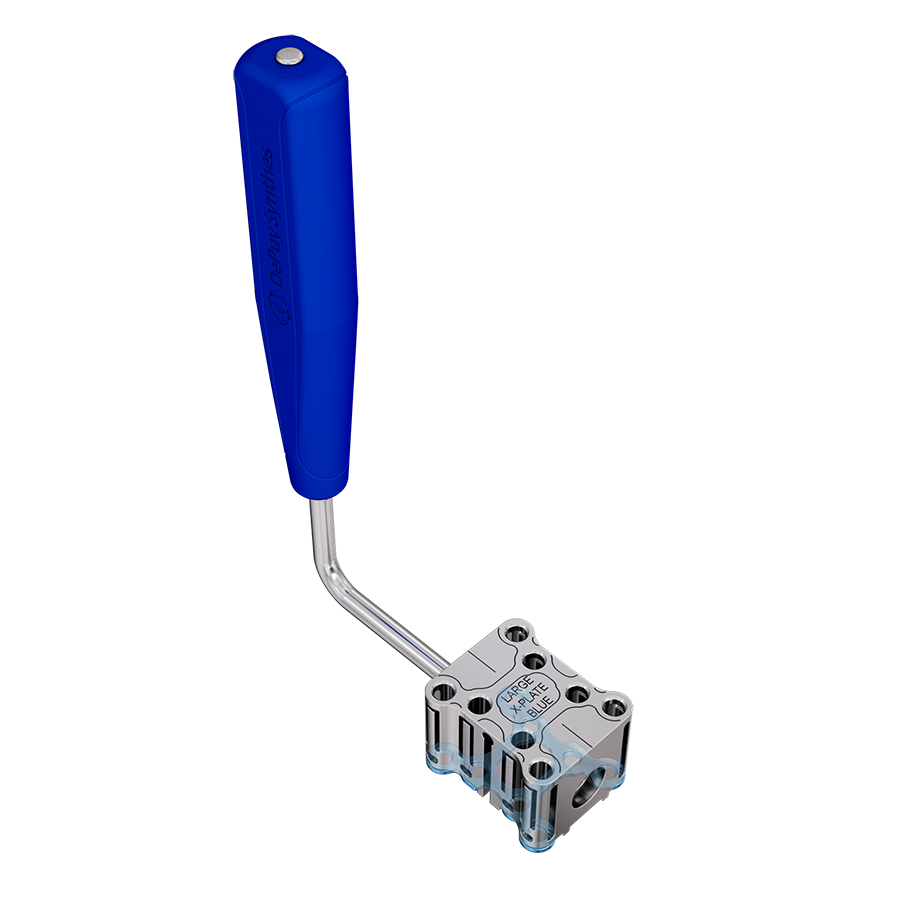
The system includes non-implantable dedicated-use Screw Guides (Fig 2 and Fig 3 within Details tab and Fig 8), a Screw Guide Handle (Fig 8), trays and modules for storage, and general use instruments to be used as accessories with the implants (Fig 9). These include Sternal Reduction Forceps (Fig 7 and Fig 9), Bending Pliers, Plate Cutters (Fig 9), and In-Situ Benders (Fig 10).
MatrixSTERNUM™ Fixation System
This session may contain graphic medical procedures and imagery intended for educational purposes. Viewer discretion is advised. The content is designed for healthcare professionals and may include sensitive or disturbing material.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.