CODA™ Anterior Cervical Plate System
Chung Chek Wong, Richard Bransford, Kazu Kitamura, Timothy Moore, Juan Zamorano, Nicolas Bronsard
Anterior cervical discectomy and fusion (ACDF) is a common surgical procedure for treating cervical spine disorders such as degenerative disc disease, herniated discs, and cervical radiculopathy. The CODA™ Anterior Cervical Plate (ACP) System was developed to improve outcomes of anterior cervical fusion surgeries.
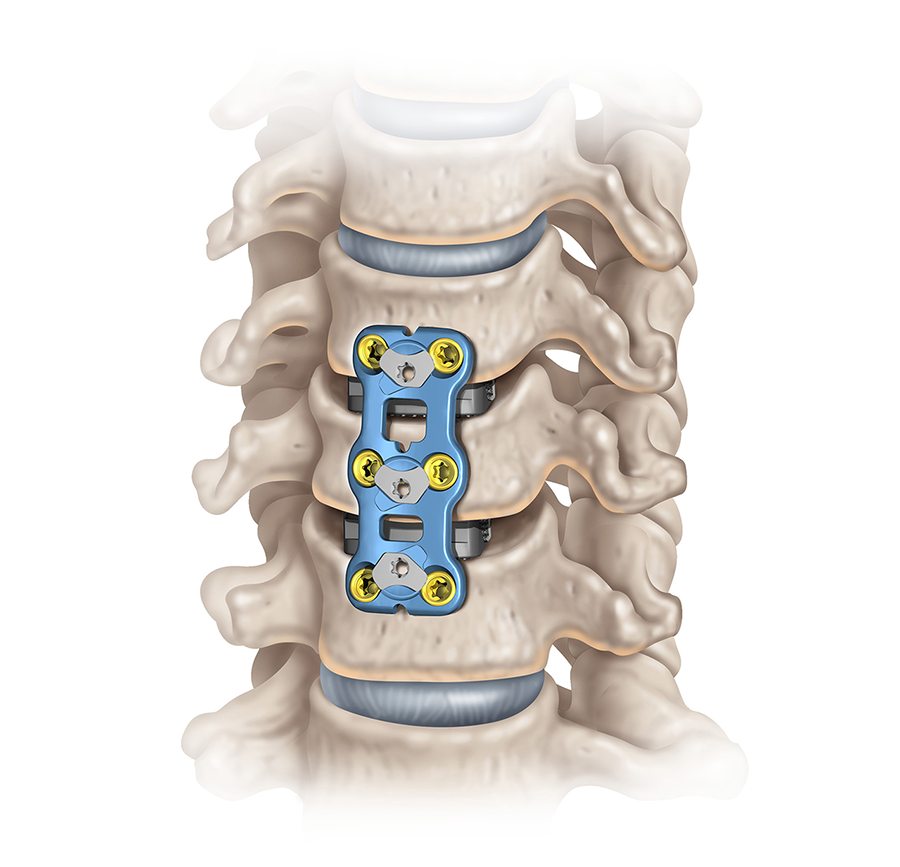
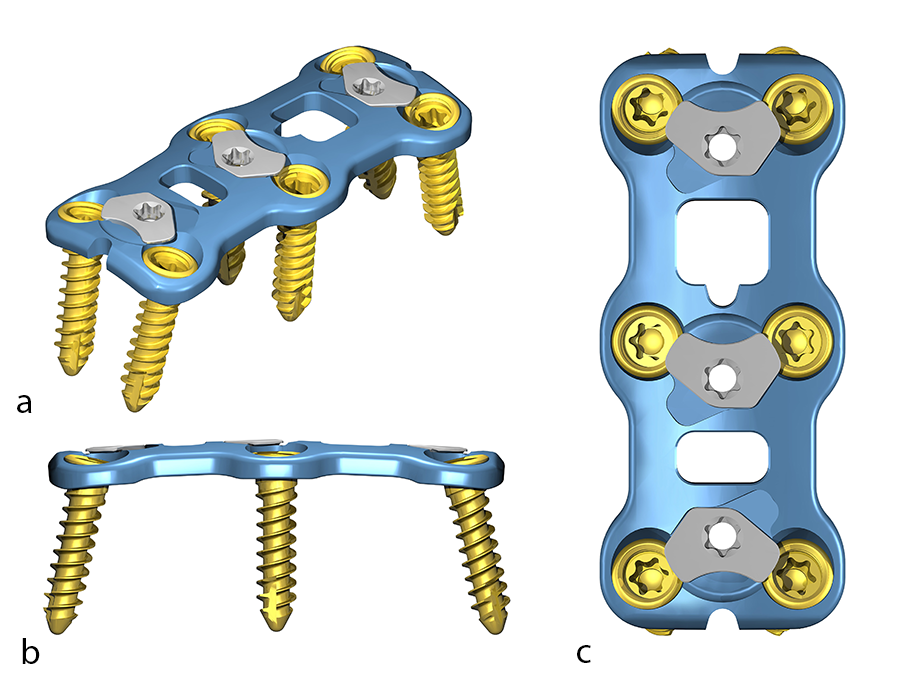
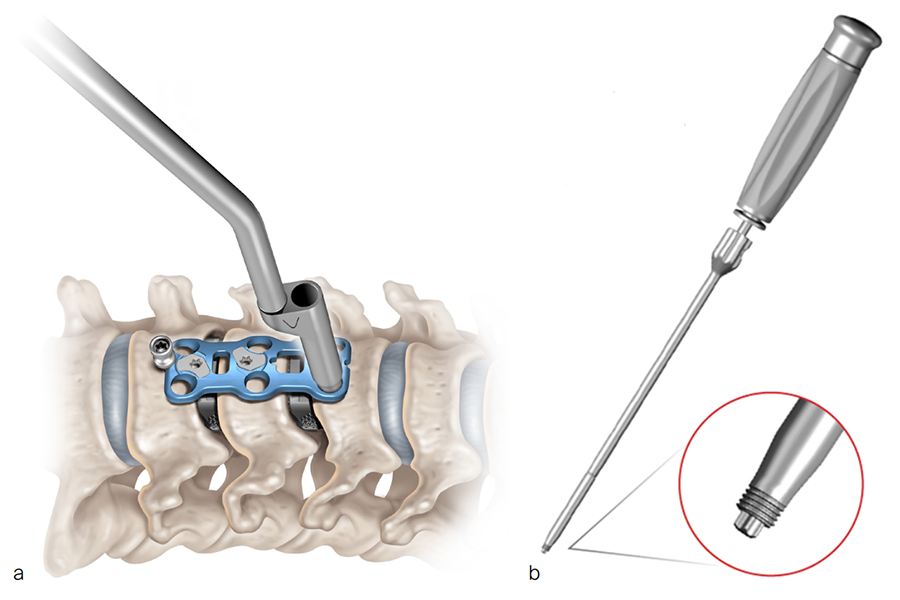
The CODA™ ACP System (Fig 1) includes low-profile titanium plates in various lengths (Fig 2 and Fig 3). The low profile is intended to reduce the incidence of dysphagia and other postoperative complications. The plates have bone grafting windows for better visibility during the surgical procedure and can accommodate variable angle and constrained angle screws with a double-lead screw thread. The combination of high screw angulation and the shorter 10 mm one-level plate could potentially reduce the incidence of adjacent vertebral-level degeneration and ossification. The plates have an integrated active locking mechanism that prevents screw loosening.
This innovative plating system potentially improves the efficiency of the surgical workflow by having one locking mechanism per level instead of one per screw. The instrumentation is streamlined, meaning that the same screwdriver can be used to insert the screw and deploy the locking mechanism. The threaded screwdriver included in the system (Fig 5b) secures the screw attachment and controls the screw during screw insertion [1].
Current clinical challenges in ACDF
Dysphagia
Dysphagia has long been recognized as a potential complication following ACDF [2, 3]. Despite its high incidence rate, the pathogenesis of this condition and associated risk factors are not well defined. A recent systematic literature review found a mean dysphagia rate of 19.4% following ACDF. Furthermore, zero-profile implants seemed to reduce dysphagia risk [2]. Therefore, the enhanced low-profile plate design of the CODA™ ACP System could offer as a key potential benefit a reduction in the incidence of dysphagia following ACDF procedures.
Adjacent segment degeneration
Although ACDF is clinically a highly successful procedure for disorders including cervical degenerative disc disease, adjacent segment degeneration (ASD) has been reported as a complication at the spinal level secondary to the rigid fixation. A recent study found that ASD occurred after single-level ACDF in 54% of cases, most commonly after C5/6 fusion (28%) [4]. The innovative design features of the CODA™ ACP System, including high screw angulation and shorter 10 mm one-level plate, may potentially reduce the occurrence of adjacent-level degeneration following ACDF.
Product details
The CODA™ ACP System consists of single-use titanium alloy (Ti-6Al-4V ELI) plates and screws that are available in both sterile and nonsterile sets. The plates are offered in various lengths and are low profile with a height of 1.9 mm (one- to three-level plates) and 2.1 mm (four- to five-level plates). The plates have bone grafting windows that allow optimal visualization of the graft, vertebral bodies, and endplates. The plates have an integrated active locking mechanism and can accommodate constrained angle and variable angle screws. The system includes nonsterile reusable instruments and sterile single-use instruments, designed to facilitate proper implantation of the plate and screws.
Plates
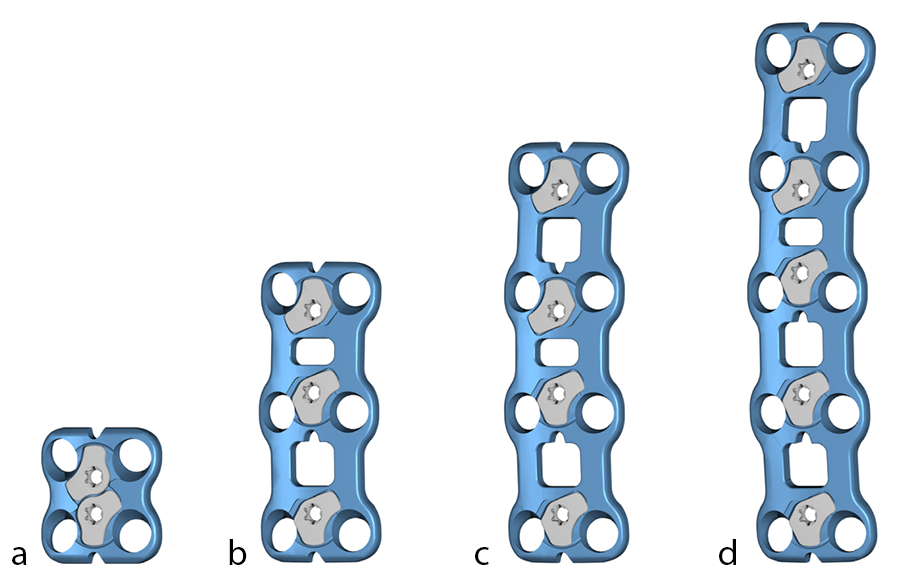
The CODA™ ACP System plates are available in these lengths (Fig 3):
- One level (lengths: 10–30 mm, increments of 2 mm)
- Two levels (lengths: 24–44 mm, increments of 2 mm)
- Three levels (lengths: 42–66 mm, increments of 3 mm)
- Four levels (lengths: 60–88 mm, increments of 4 mm)
- Five levels (lengths: 75–105 mm, increments of 5 mm)
The plate profile is 1.9 mm in 1–3-level plates, and 2.1 mm in 4–5-level plates. The screw locking mechanism is flush to the plate. The plate width is 17 mm at the widest point and 13 mm at the waist.
Screws
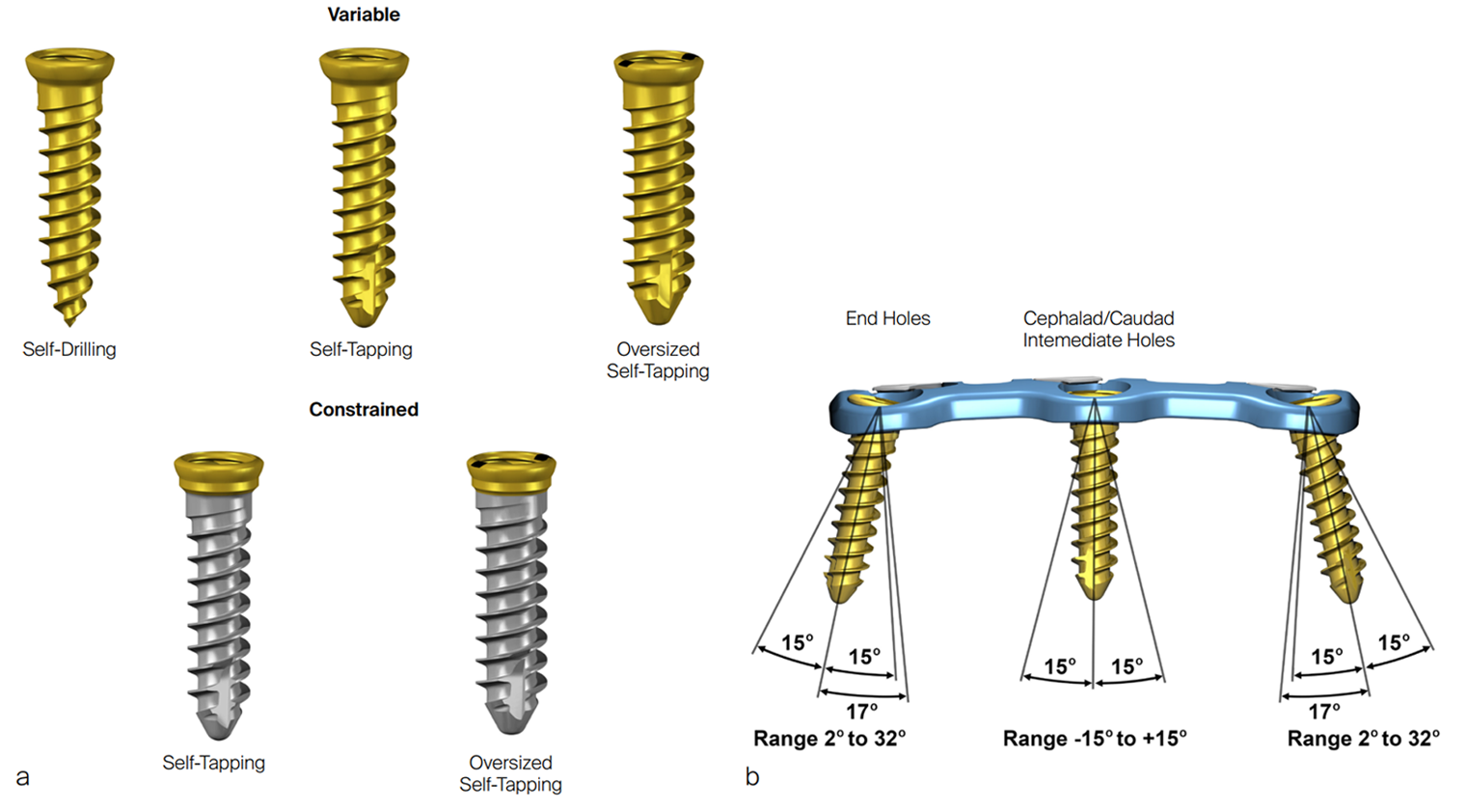
The screws (Fig 4) are available as both sterile and nonsterile and feature a double-lead screw thread. Variable angle screws are available as self-drilling Ø3.5 mm screws (lengths: 10–18 mm), self-tapping Ø3.5 mm screws (lengths: 10–18, 20, and 22 mm) and self-tapping Ø4.0 mm screws (lengths: 12, 14, 16, and 18 mm). The plate screw holes permit a screw angulation of ±15° with cephalad/caudad range end holes from 2° to 32°.
Constrained angle screws are available as both self-tapping Ø3.5 mm screws (lengths: 10–18, 20, and 22 mm) and self-tapping Ø4.0 mm screws (lengths: 12, 14, 16, and 18 mm).
Key features and benefits
Patients:
- A low profile plate may potentially reduce incidence of dysphagia.
- High screw angulation and shorter 10 mm one-level plate may potentially reduce occurrence of adjacent-level degeneration.
Surgeons/healthcare professionals:
- Efficiency of surgical workflow is enhanced by having one locking mechanism per level instead of per screw. The positive stop on the locking mechanism is consistent for all plate levels.
- The same T10 screwdriver can be used to insert screws and deploy the locking mechanism.
- The threaded screwdriver provides secure attachment and control during screw insertion.
Indications (United States only)
The CODA™ ACP System is intended for anterior fixation of the cervical spine (C2–7) as an adjunct to fusion in skeletally mature patients. Specific indications include degenerative disc disease (defined as neck pain of discogenic origin with degeneration of the disc confirmed by history and radiographic studies), spondylolisthesis, trauma (ie, fracture or dislocation), spinal stenosis, deformities or curvatures (ie, scoliosis, kyphosis, and/or lordosis), tumor, pseudarthrosis, and failed previous fusion.
Contraindications (United States only)
In adults, contraindications of the CODA™ ACP System and spinal fixation surgery include:
- Active systemic infection or an infection localized to the site of the proposed implantation, including presence of fever
- Severe osteoporosis which may prevent adequate fixation of screws and thus preclude the use of this or any other spinal instrumentation system
- Patients who have been shown to be safely and predictably treated without internal fixation
- Open wounds
- Relative contraindications include any entity or condition that totally precludes the possibility of fusion (eg, cancer, kidney dialysis, or osteopenia), obesity, alcoholism or drug abuse, smoking, pregnancy, mental illness, certain degenerative diseases, and foreign body sensitivity.
References
- Resolve Surgical Technologies. Usability Test Report #2277, Revision 2, Jan 17, 2023.
- Tsalimas G, Evangelopoulos DS, Benetos IS, et al. Dysphagia as a Postoperative Complication of Anterior Cervical Discectomy and Fusion. Cureus. 2022 Jul 15;14(7): e26888.
- Carucci LR, Turner MA, Fitzhugh Yeatman C. Dysphagia Secondary to anterior cervical fusion: radiologic evaluation and findings in 74 patients. AJR Am J Roentgenol. 2015 Apr;204(4):768–775.
- Alhashash M, Shousha M, Boehm H. Adjacent Segment Disease After Cervical Spine Fusion: Evaluation of a 70 Patient Long-Term Follow-Up. Spine. 2018 May 1;43(9):605–609.
Instruments
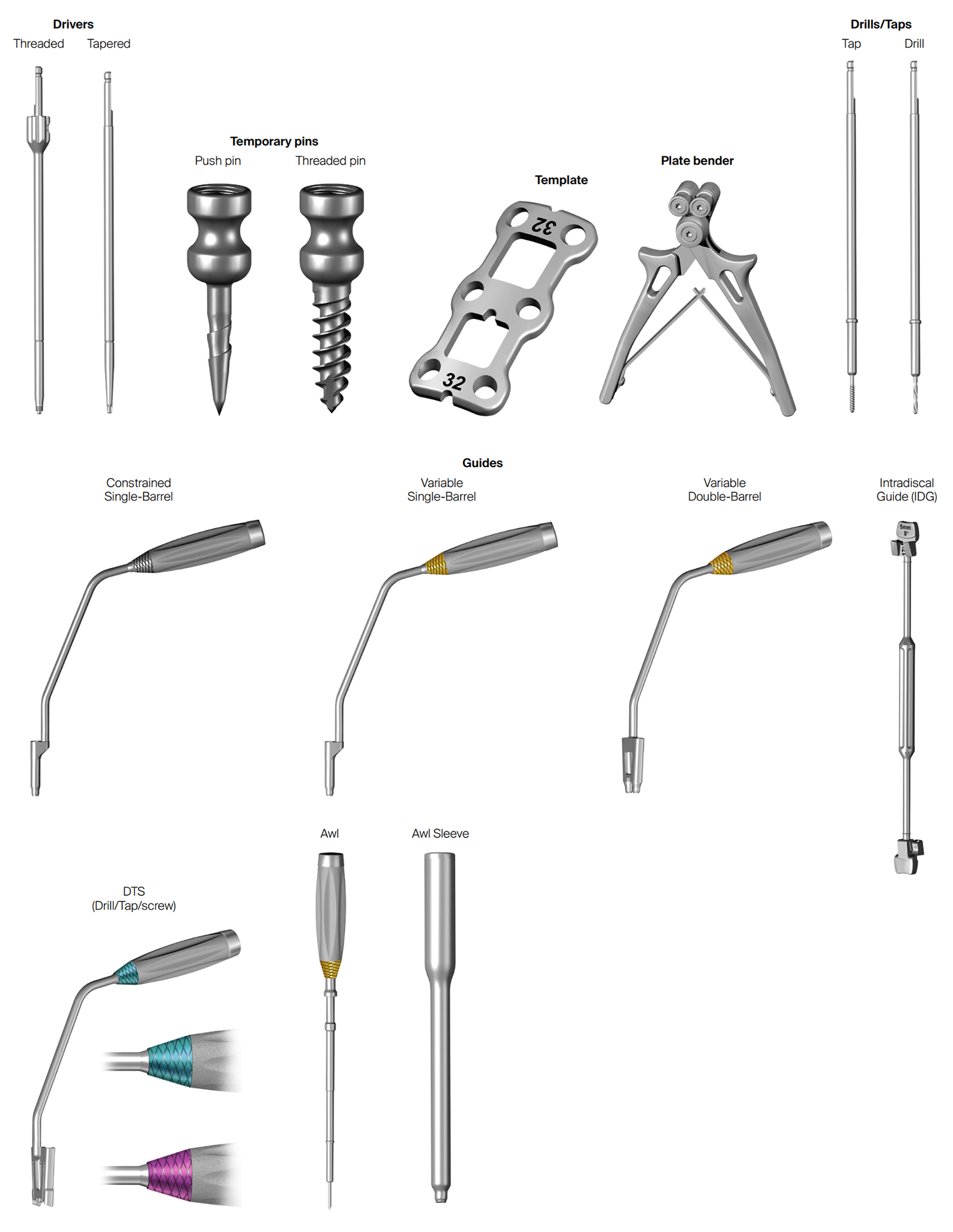
Instruments included in the CODA™ ACP System include drivers (threaded and tapered), temporary pins for fixation (push pins and threaded pins), templates, plate bender, awl, drills, taps, and screw guides (Fig 5 and Fig 6).
Cases kindly provided by Rick Bransford, Harborview Medical Center, Seattle
Case 1: 46-year-old man with Central Cord Syndrome following a fall down stairs
A 46-year-old man presented for evaluation of the cervical spine with magnetic resonance imaging (MRI) evidence of C4/5 mild retrolisthesis with central cord compression.
Initial injury: February 8, 2024
The patient tripped and fell down three stairs on February 8, 2024, landing face-first onto a cement floor. He was noted to have deficits to all limbs at the time of injury, with decreased grip strength. He denied neck or back pain. Magnetic resonance imaging and CT scans were performed, demonstrating C4/5 retrolisthesis, and no acute fractures but evidence of central stenosis at C4/5. The patient was offered surgery at the time of initial hospital admission but declined so that he could return to family in the US.
Assessment at spine clinic: February 26, 2024
The same patient presented at the spine clinic for evaluation of the cervical spine. The chief complaint was of bilateral upper extremity weakness and hyperesthesia affecting shoulder to fingers bilaterally. The patient reported dexterity problems in his hands and the inability to hold small objects, such as a pen. He also reported pain radiating bilaterally from elbow to fingers. The patient was not engaged in formal physical therapy. Without medication, the patient reported pain of 6/10. No relevant past medical or surgical history was reported.
Physical examination of the upper extremities showed bilateral muscle weakness (elbow, wrist, and hand), and areas of hyperesthesia on the left side.
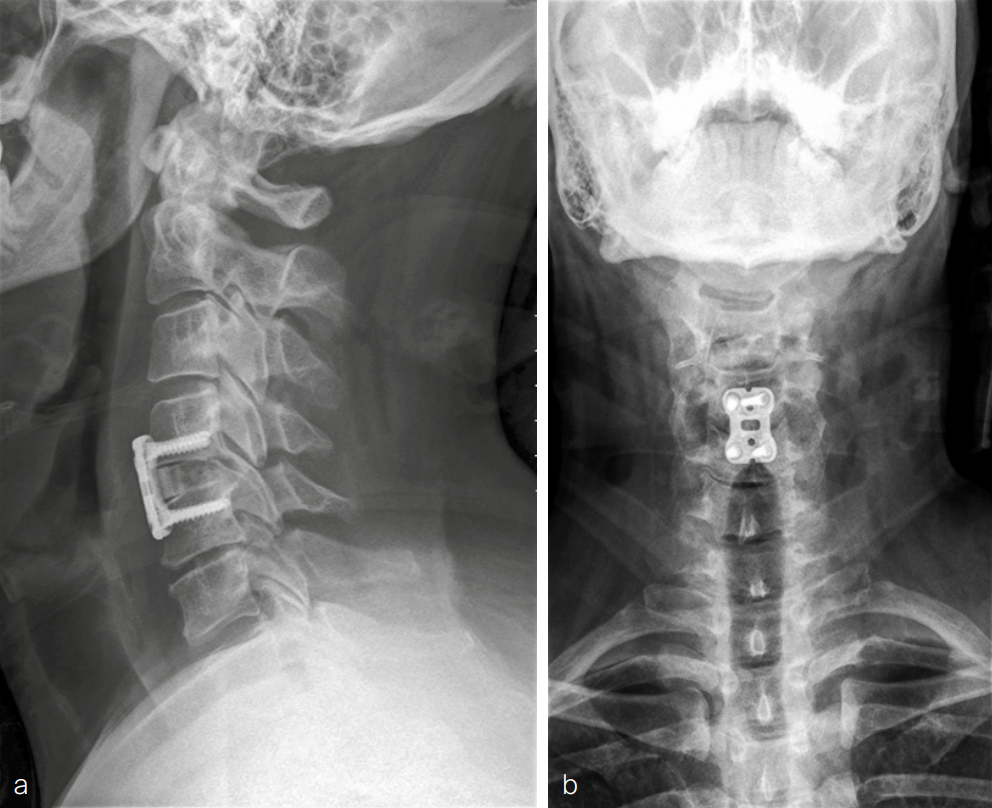
Imaging was conducted. Plain x-rays (Fig 7) showed:
- No acute fracture
- Evidence of degenerative disc disease at levels C5/6 with focal disc height loss.
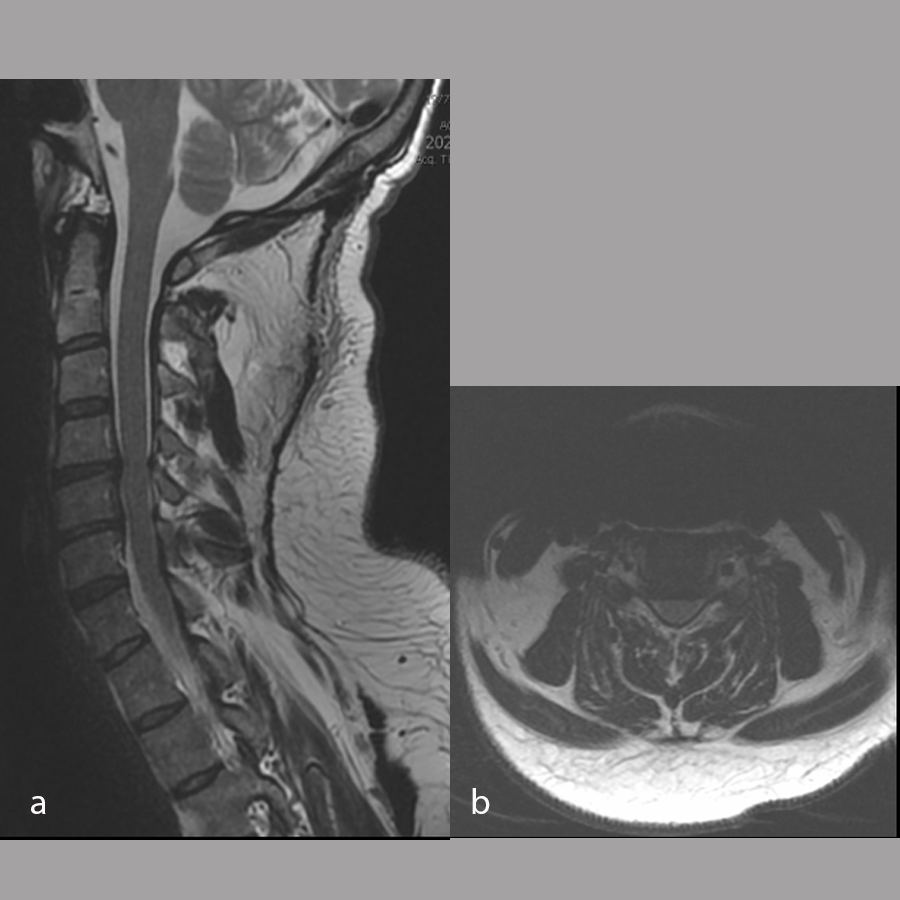
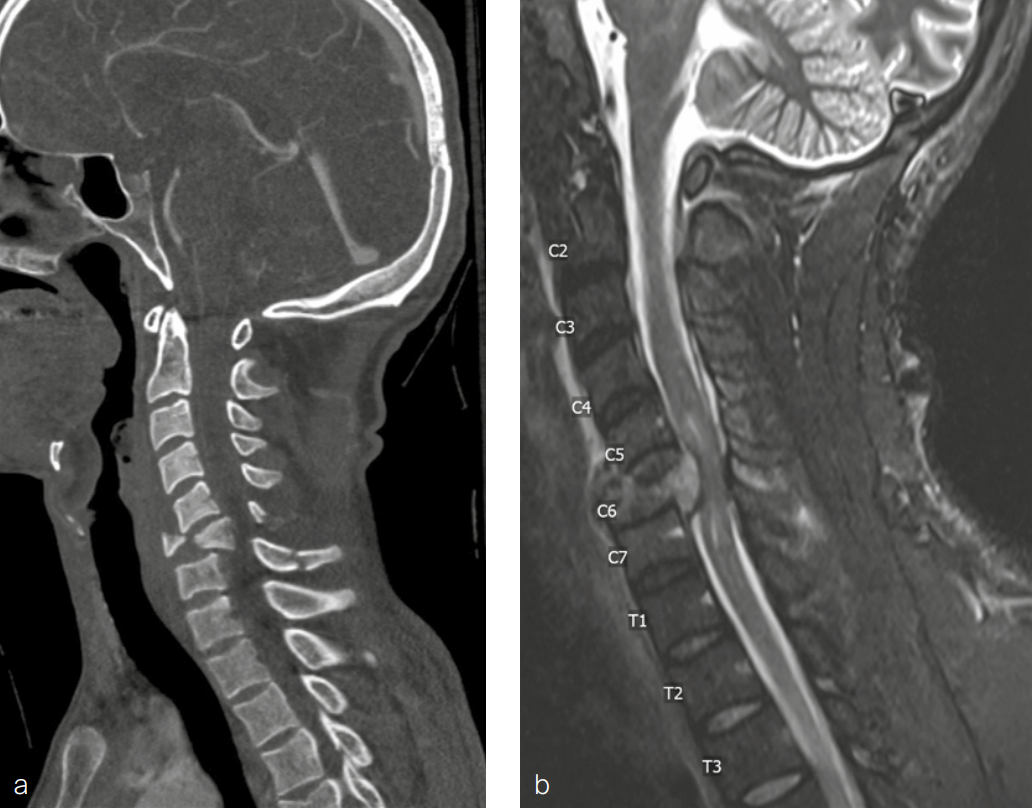
Magnetic resonance imaging of the cervical spine performed at the time of injury (Fig 8) was reviewed and showed:
- Spinal canal narrowing at C4/5 with cord compression and associated cord edema
- Bilateral uncovertebral osteophytes with mild bilateral foraminal narrowing
- C5/6 with mild posterior disc bulging causing mild canal narrowing without cord compression
- Mild bilateral foraminal narrowing of C6/7.
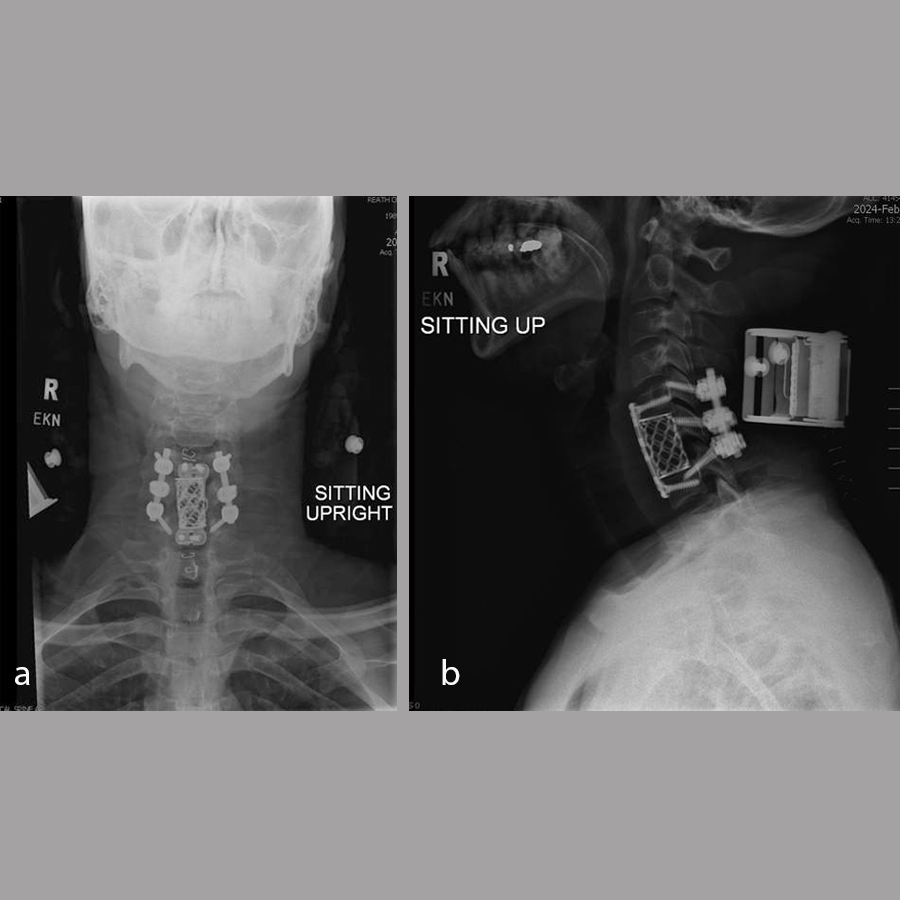
A diagnosis was made of cervical spinal stenosis largely attributable to stenosis at C4/5, with lesser stenosis at C5/6 and cord compression causing sub-acute central cord syndrome. Anterior decompression was recommended to alleviate symptoms, and C4/5 anterior cervical discectomy and fusion (ACDF) was performed on March 7, 2024. Fig 9 shows postoperative imaging.
At 6-month follow-up, muscle strength was greatly improved with physical examination showing 5 out of 5 strength throughout both upper extremities. Sensation was normal in both upper extremities. The patient had a good range of motion in the cervical spine. He continued to have some problems with fatigue whilst handwriting. There was some residual pain and neuropathy bilaterally, in a small area from the mid-forearm to the mid-bicep. The nerve symptoms tended to fluctuate throughout the day. Continued improvement was anticipated with ongoing physical therapy.
Anteroposterior and lateral, flexion, and extension x-rays showed that the graft at C4/5 appeared to be in the original position as expected. There was no evidence of loosening of the screw-plate construct. Some bridging fusion was present between the superior C5 endplate of the graft. There continued to be incomplete consolidation between C4 and the graft but no motion of the graft itself. Flexion/extension films did not show any instability.
Case 2: 34-year-old man injured following fall from 10-m height
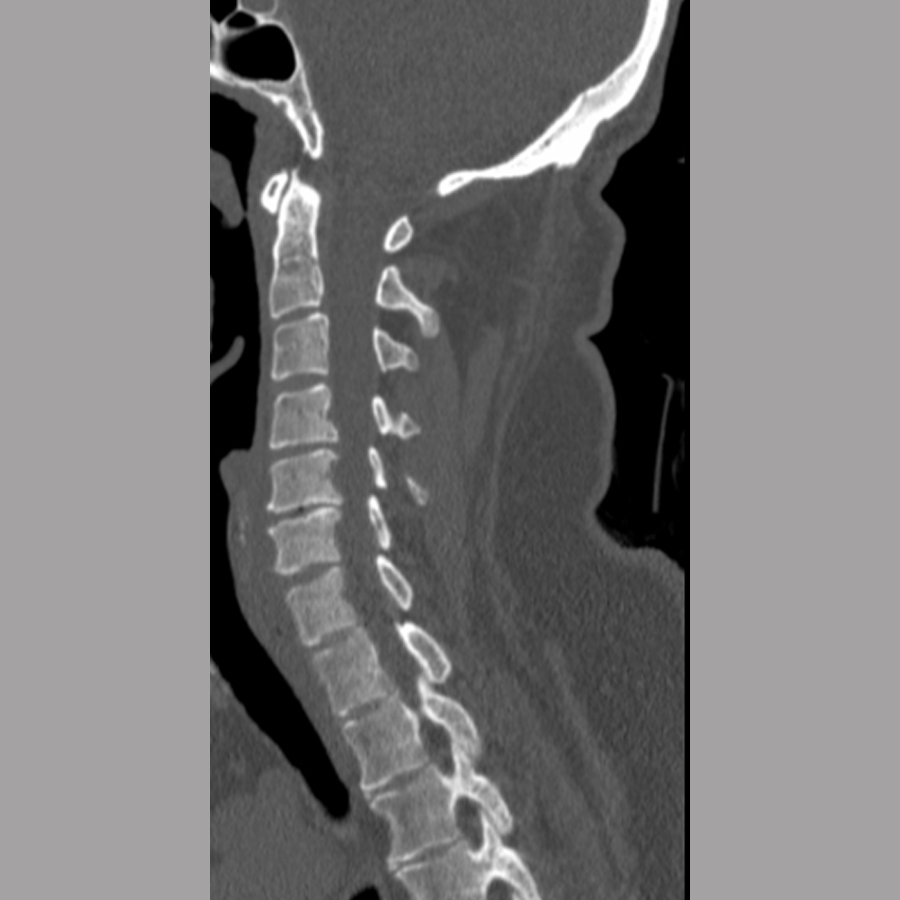
A 34-year-old man with a history of alcohol use disorder and prior seizures was admitted to hospital after being found outside his house having reportedly fallen from a second-story window. The patient had numbness and left-sided deficits, did not recall the circumstances of the fall, and was very intoxicated in the emergency department.
Full-spine imaging (Fig 10) showed injuries including a C6 flexion distraction with significant burst fracture (AO C6 B2 A4) with retropulsion into the canal and associated kyphosis, with an associated mildly displaced C6 spinous process fracture, C7 displaced spinous process fracture, occipital condyle fractures without extension into the facets, and transverse process fractures of T11, L1, L2, L3, and L4.
Other injuries included fractures of ribs 10–12 on the left side, left pneumothorax and lung contusion, left vertebral artery occlusion, and hemorrhagic contusions of frontal lobes and temporal lobes.
Physical examination showed muscle weakness in the left upper extremity, especially in the hand, and reduced sensation in the left upper extremity (C5–T1). The lower extremities had normal muscle strength and sensation.
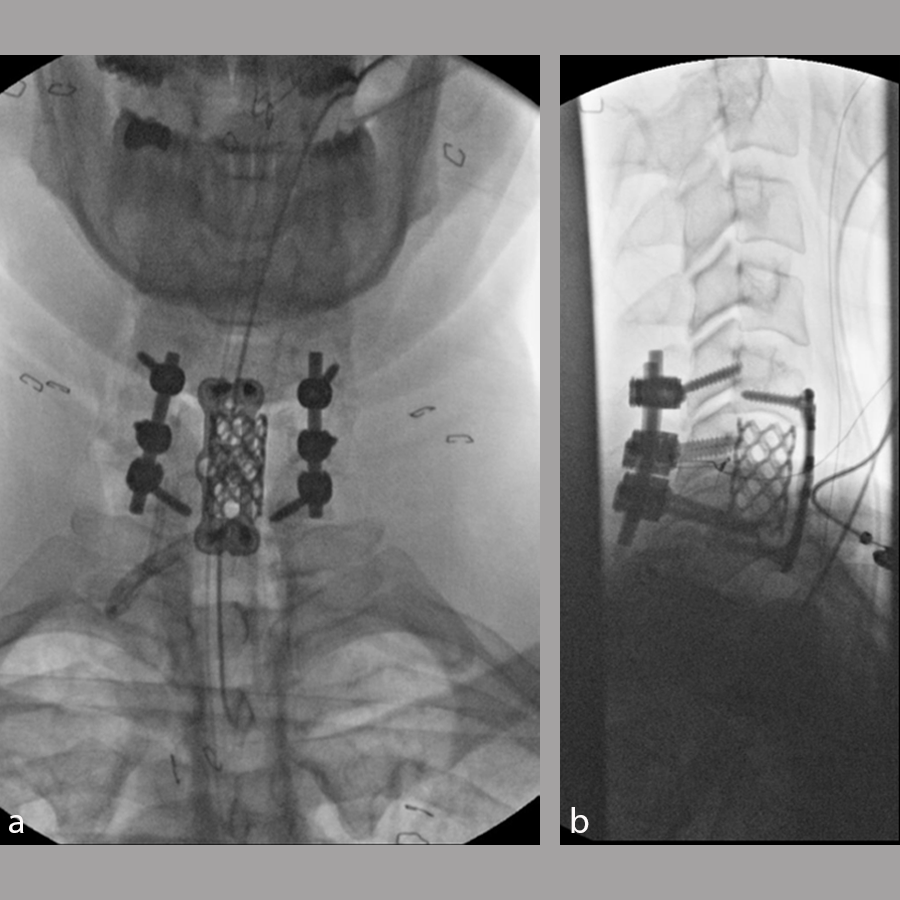
The patient underwent a C6 corpectomy with removal of fractured C6 vertebral body, restoration of height and placement of a Synmesh cage filled with autograft from the fracture. Following restoration of height and vertebral body reconstruction, a CODA™ ACP plate was applied anteriorly with screws into the C5 and C7 vertebral bodies. Given the psychosocial nature of the patient and the fracture pattern, the patient was then placed prone and underwent posterior instrumentation with Symphony instrumentation with lateral mass screws into C5 and C6 bilaterally and pedicle screws into C7 followed by placement of 4 mm rods along with fusion (Fig 11). The patient did well postsurgery (Fig 12) and was discharged according to protocol, but was unfortunately then lost to follow-up.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.