TELIGEN™ System
Peter Derman, So Kato, Matti Scholz
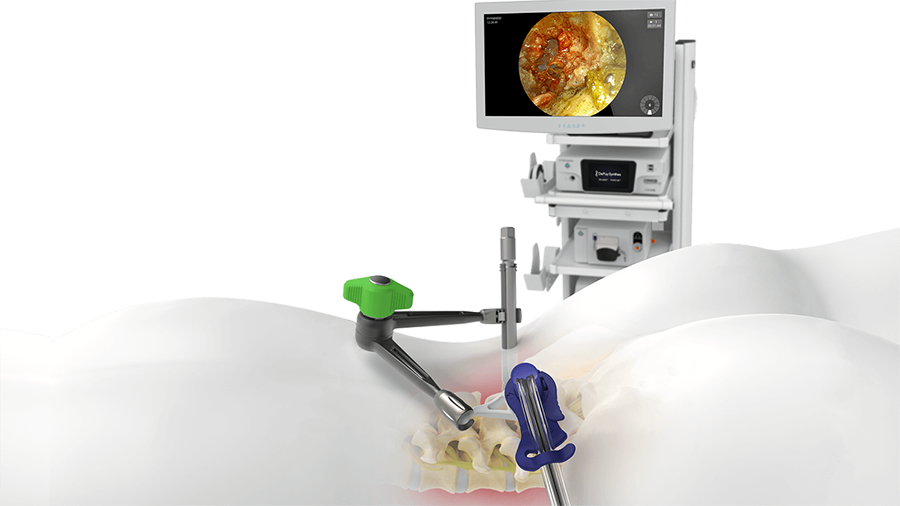
Minimally invasive surgical transforaminal lumbar interbody fusion (MIS-TLIF) is typically performed using tubular retractors with visualization aided by surgical loupes or a microscope. More recently, endoscopic TLIF has been introduced. In addition to reducing surgical morbidity, endoscopic techniques place the camera within the surgical wound. The angled optics enable the surgeon to "see" around corners which is beneficial when working in the constrained space of a minimally invasive procedure.
In November 2022, the AO Spine Technical Commission approved the TELIGEN™ System, which is indicated for facilitating endoscopic access and visualization in spinal procedures and aims to combine the positive attributes of tubular retractors and endoscopy. It provides surgeons skilled in MIS-TLIF with the advantages of endoscopic TLIF (eg, wide-angle, directed optics placed near the anatomical structures) without the difficult learning curve associated with adopting a completely new skill set.
Clinical problem
Transforaminal lumbar interbody fusion (TLIF) is a common surgical technique performed via a posterior approach with favorable fusion rates [1]. Open TLIF, however, can present clinical challenges, including greater soft-tissue disruption [1], increased complication risk [2−5], slower recovery [6, 7], and variable long-term outcomes, such as greater back pain and disability [7].
The MIS-TLIF techniques have evolved to reduce complications seen with open TLIF [1]; however, they may be linked with certain limitations, such as lack of consistent visualization and steep learning curve [8], and occupational hazards to the surgeon [9,10].
Solution
TELIGEN™ System is a technology platform that facilitates MIS-TLIF procedures through digital tools for visualization and access. Its first clinical application is a beginning-to-end procedural solution for MIS-TLIF surgery (DePuy Synthes VueLIF™-T Procedure). Other enabling technologies, like neuromonitoring and navigation, can be integrated into the workflow.
TELIGEN™ System overview/instruments
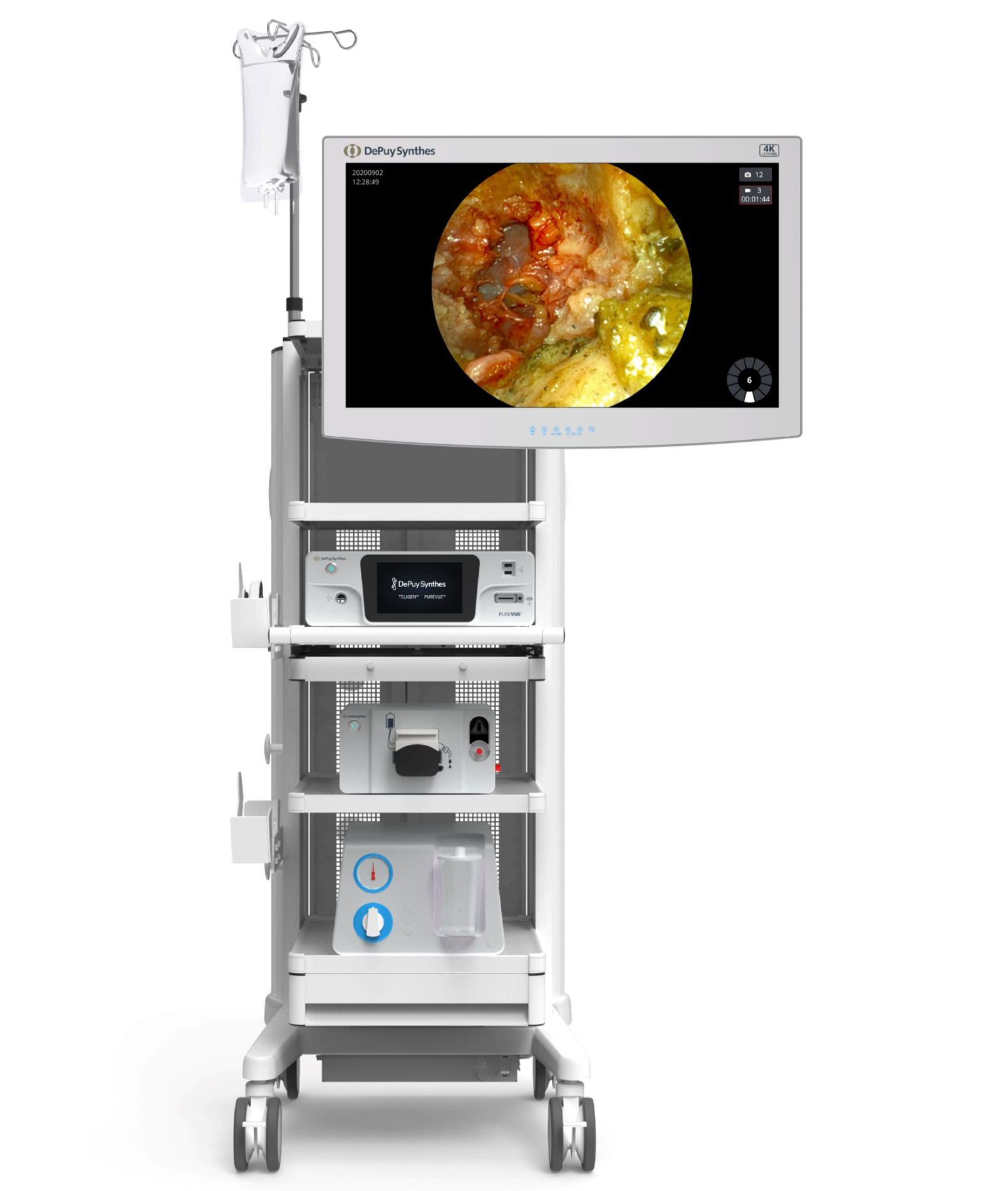
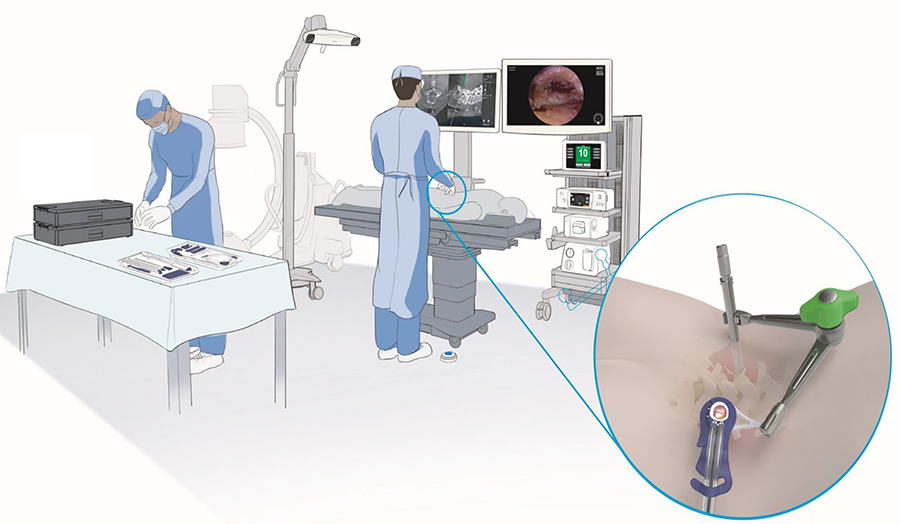
The system components are centrally controlled from the TELIGEN Digital Control Center (tower). Included in the tower are the monitor, the camera control system (CSS), the camera wash box with foot pedal control, and the vacuum pump for discectomy (Fig 1).
The TELIGEN HD CCS operates the TELIGEN™ Camera, which is used for illumination and visualization of the surgical site. The image collected at the TELIGEN™ Camera is transferred to the CCS and then displayed on the monitor.
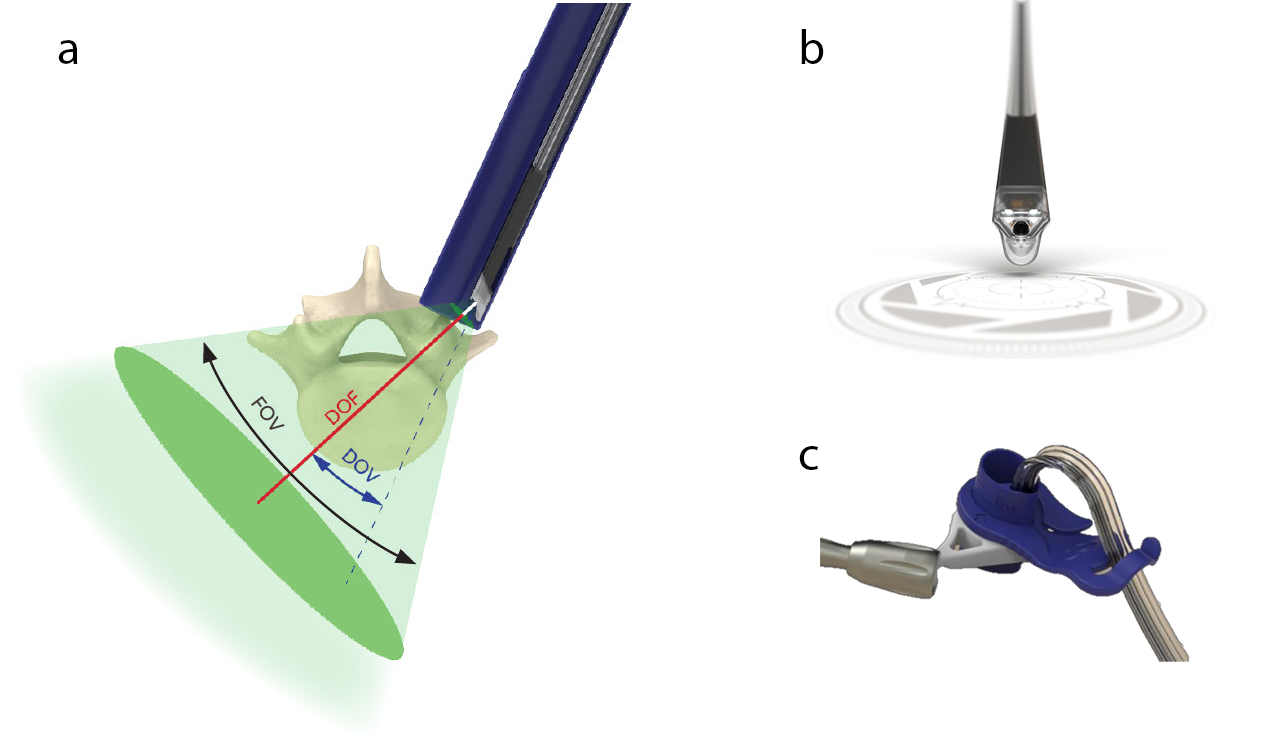
The TELIGEN™ Camera provides an opportunity to see beyond the boundaries of the port. Placing the camera at the distal end of the port enables line of sight past the edge of the port, expanding the field of view of the surgical site. Visual interference from the surgical instruments is reduced compared to traditional visualization methods (eg, surgical microscope, loupes, exoscope). The camera has a field of view (FOV) of 70°, direction of view (DOV) of 22.5°, and a depth of field (DOF) of 8−120 mm (Fig 2).
Two LEDs located near the tip of the camera provide light across the field of view. The camera is self-retained in the channel of the port allowing for hands-free use. The camera cable is held by the cable wing of the port holder, securing the camera’s position, and preventing interference within the surgical workspace. This allows for bimanual control of instruments during the procedure.
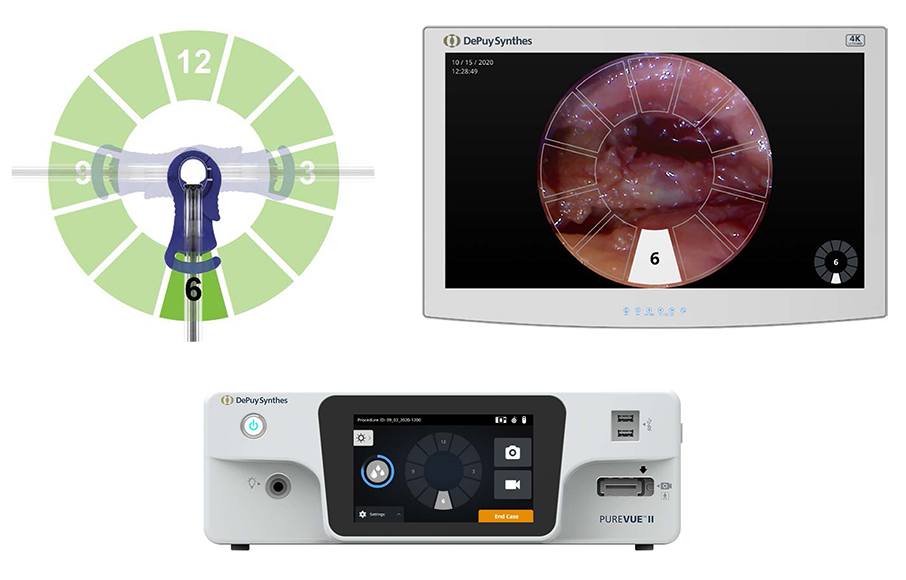
The camera view can be rotated around the access port axis during the procedure. After the camera is rotated within the patient, pressing the corresponding clock position on the CCS’s user interface will match the on-screen orientation (Fig 3).
The TELIGEN™ Camera Wash Box assists the surgeon in maintaining a clear visual field during endoscopic spine surgery by cleaning the lens of the camera. The camera lens and surgical site can be irrigated in-situ without removal from the working channel. The cleaning cycle is activated by foot pedal or from the CCS touchscreen.
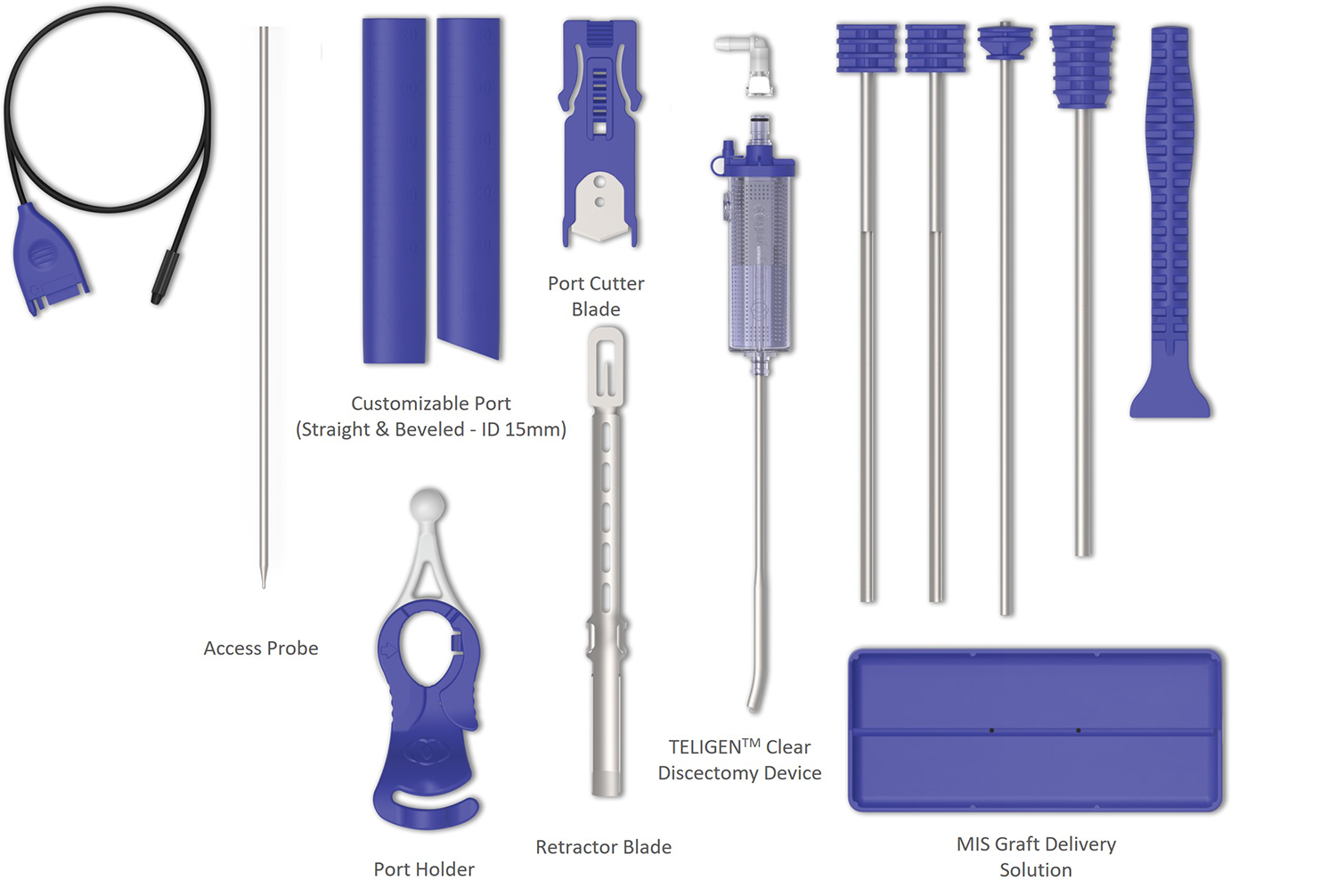
TELIGEN™ comes with a disposable procedure kit and a set of reusable instruments. The contents of each of these sets may vary in some markets. These components are designed to facilitate the entire procedure beginning to end introducing procedural efficiencies along with features that are complimented using the TELIGEN™ camera.
VueLIF-T™ Procedure Kit contains sterile, single-use instruments to allow for access, visualization, discectomy, and graft delivery. It includes the camera, ports, and port holder, TELIGEN™ Clear (MIS discectomy device, former CONCORDE Clear), soft-tissue retractor, port cutter cartridge, and bone graft delivery instruments (Fig 4).
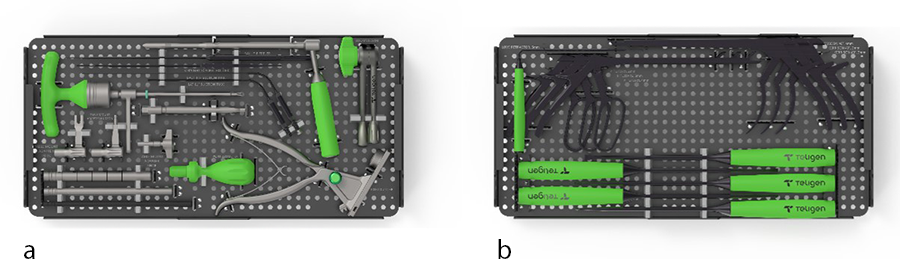
The set of reusable instruments include table mounting instruments, dilators, port adjuster and port cutter, graft delivery cannula, suction tubes, and Kerrisons, etc (Fig 5).
The head-up display allows surgeons to maintain ergonomic posture during procedures (Fig 6) [11]. This may help avoid significant musculoskeletal pain commonly reported by spine surgeons, especially low back and neck pain [12,13].
Conclusion
TELIGEN™ System provides an advanced visualization experience and will first be applied to MIS-TLIF. The system is intended to provide minimally invasive access to the spine without the difficult learning curve associated with the adoption of full endoscopic surgery.
The heads-up display allows surgeons to maintain an ergonomic posture during the procedure. Furthermore, this visualization and display facilitates teaching of trainees as well as engagement by operating room personnel, potentially increasing operative efficiency.
TELIGEN™ System was thoroughly tested and approved by the Lumbar Degenerative Expert Group (Fig 7).
References
- Mobbs RJ, Phan K, Malham G, et al. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg. 2015 Dec;1(1):2−18.
- Parker SL, Adogwa O, Witham TF, et al. Post-operative infection after minimally invasive versus open transforaminal lumbar interbody fusion (TLIF): literature review and cost analysis. Minim Invasive Neurosurg. 2011 Feb;54(1):33−37.
- Tan JH, Liu G, Ng R, et al. Is MIS-TLIF superior to open TLIF in obese patients?: A systematic review and meta-analysis. Eur Spine J. 2018 Aug;27(8):1877−1886.
- Xie Q, Zhang J, Lu F,et al. Minimally invasive versus open transforaminal lumbar interbody fusion in obese patients: a meta-analysis. BMC Musculoskelet Discord. 2018 Jan 17;19(1):15.
- Othman YA, Alhammoud A, Aldahamsheh O, et al. Minimally invasive spine lumbar surgery in obese patients: a systematic review and meta-analysis. HSS J. 2020 Jul;16(2):168−176.
- Hockley A, Ge D, Vasquez-Montes D, et al. Minimally invasive versus open transforaminal lumbar interbody fusion surgery: an analysis of opioids, nonopioid analgesics, and perioperative characteristics. Global Spine J. 2019 Sep;9(6):624−629.
- Xie L, Wu WJ, Liang Y. Comparison between minimally invasive transforaminal lumbar interbody fusion and conventional open transforaminal lumbar interbody fusion: an updated meta-analysis. Chin Med J (Engl). 2016 Aug 20;129(16):1969−1986.
- Simpson AK, Lightsey HM 4th, Xiong GX, et al. Spinal endoscopy: evidence, techniques, global trends, and future projections. Spine J. 2022 Jan;22(1):64−74.
- Stucky CH, Cromwell KD, Voss RK, et al. Surgeon symptoms, strain, and selections: systematic review and meta-analysis of surgical ergonomics. Ann Med Surg (Lond). 2018 Mar;27:1−8.
- Naresh-Babu J, Arun-Kumar V, Raju DGS. Surgeon's neck posture during spine surgeries: the unrecognised potential occupational hazard. Indian J Orthopaed 2019 Nov-Dec;53(6):758−762.
- DePuy Synthes. Letter to file─Teligen Ergonomic Posture. November 12, 2021. DePuy Quality Management System, adaptive No. 103862143.
- Swank KR, Furness JE, Baker E,et al. A survey of musculoskeletal disorders in the orthopaedic surgeon: identifying injuries, exacerbating workplace factors, and treatment patterns in the orthopaedic community. J Am Acad Orthop Surg Glob Res Rev. 2022 May 1;6(5):e20.00244.
- McQuivey KS, Deckey DG, Christopher ZK, et al. Surgical ergonomics and musculoskeletal pain in orthopaedic surgery residents: a multicenter survey study. J Am Acad Orthop Surg Glob Res Rev. 2021 Mar 11;5(3):e20.00119.
(Case provided by Michael Y Wang, MD, University of Miami Hospital and Sasha Vaziri, MD, Sarasota, Florida, USA)
- A 73-year-old woman
- Severe and worsening low back pain, left lower extremity numbness, and radiculopathy
- Failed multiple rounds of conservative management including epidural steroid injections, physical therapy, and medications
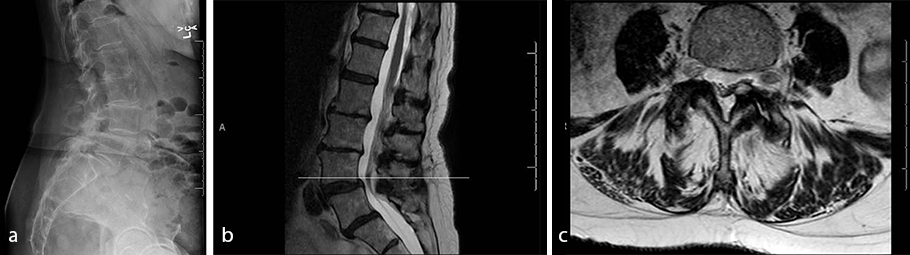
- Imaging revealed L4/L5 spondylolisthesis and a left-sided synovial cyst (Fig 8)
- Diagnosis: lumbar spondylolisthesis and radiculopathy
Surgical treatment
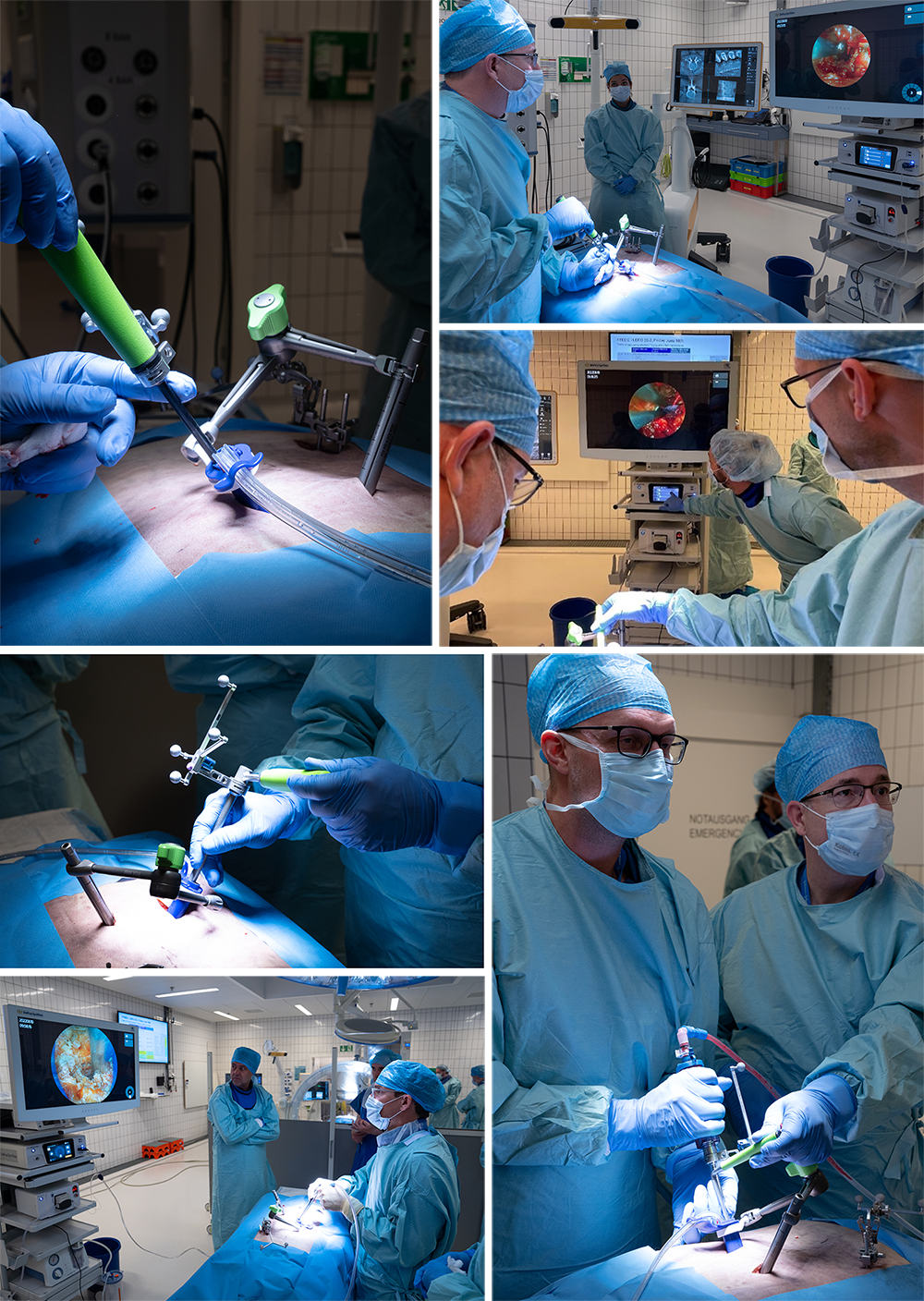
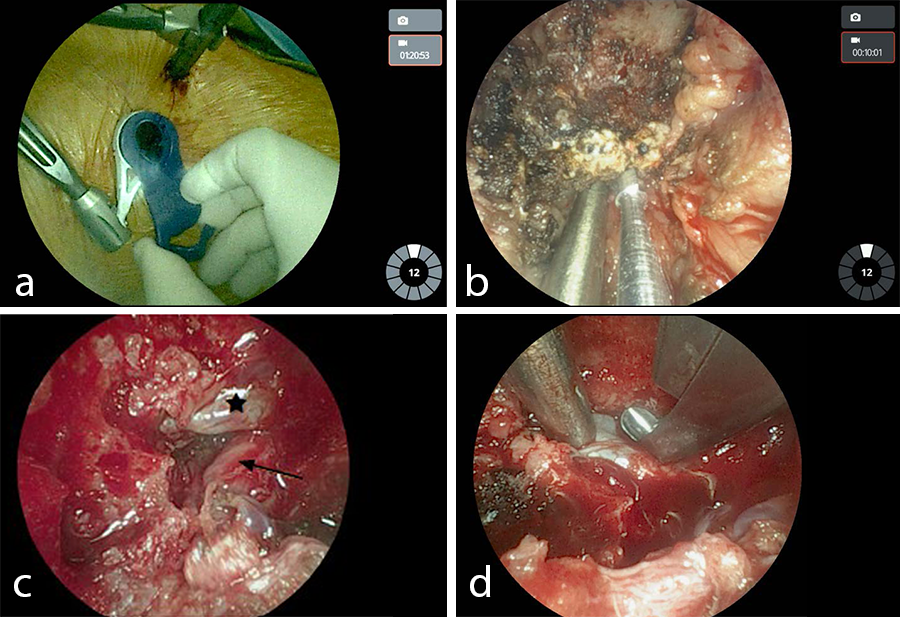
- Left L4/L5 VueLIF™ Procedure utilizing the TELIGEN™ System
- Patient-mounted TELIGEN™ tubular access system using a MIS-TLIF approach (Fig 9)
- Discectomy with TELIGEN™ Clear
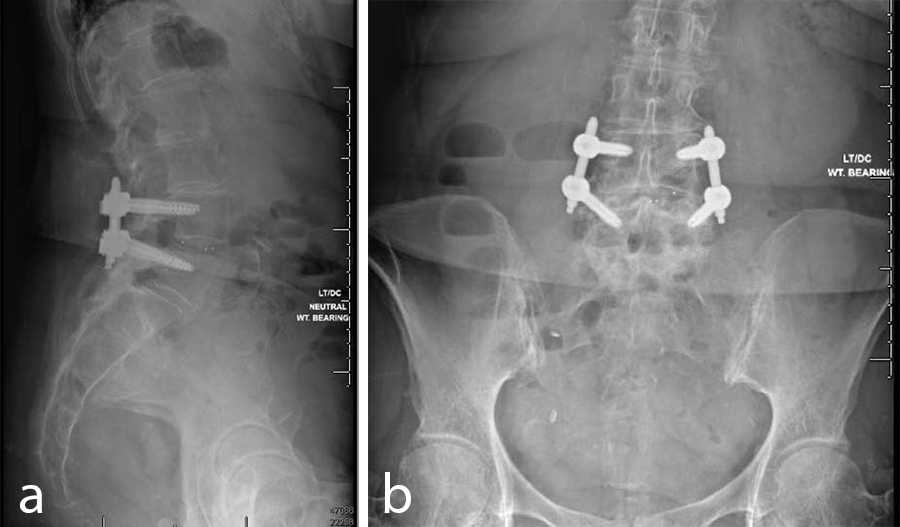
- VIPER PRIME™ Screws and CONCORDE™ Bullet Interbody Cage (Fig 10)
Outcomes
- The patient did well and stayed one night at the hospital.
- At the 6-week postoperative follow-up she was pain free.
A new look into minimally invasive TLIF - VueLIF™ procedure with the TELIGEN™ System
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.