3.5 mm Intrapelvic Acetabular System
Michael D Stover, Jorge D Barla, Robin E Peter, Ramesh K Sen, David Stephen
Due to demographics and an increasingly aging population, pelvic fractures are becoming highly relevant for society and the healthcare system. Moreover, with a shift from nonoperative to operative treatment, the number of surgical procedures worldwide is expected to grow by more than 30% [1, 2]. Nowadays, sustained physical or even sports activity is an inherent factor of the quality of life in this population. Consequently, medium- to low-energy fractures, such as pelvic ring or acetabulum fractures, are more frequent.
Similarly, there are trends within acetabulum fractures of the elderly to include specific fracture patterns and their variants. These include associated both column fractures, anterior column posterior hemitransverse, and anterior column variants. These fracture patterns will have fracture components that include the quadrilateral surface and a portion of the adjacent joint. Screw fixation alone may not be sufficient in some of these patterns because of bone quality. A plate applied to the quadrilateral surface may be required.
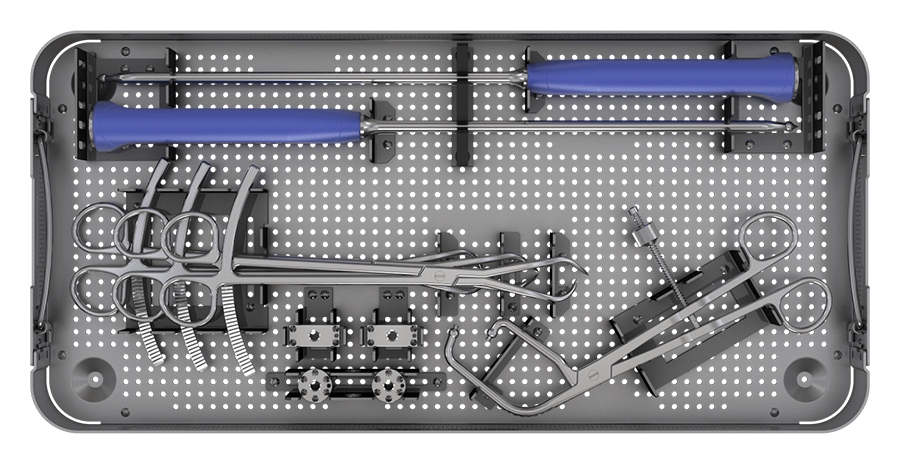
These trends define a need for new intrapelvic acetabular devices to be used for stabilization of these acetabular fractures. The possibility to deploy such new devices while using less invasive surgical approaches is of significant benefit to more fragile patients. The solution is to provide a biomechanically sound implant set that can be adjusted intraoperatively to match the patient's anatomy. Further, a new complete set of instruments including plate trials and plateholders, retractors, reduction forceps, benders, and tools for screw insertion will provide superior ease of use compared with other available solutions.
The system was designed taking into consideration the increasing popularity of the anterior intrapelvic approach and techniques for plate fixation of these fracture types. Emphasis was put on reducing surgical complexity.
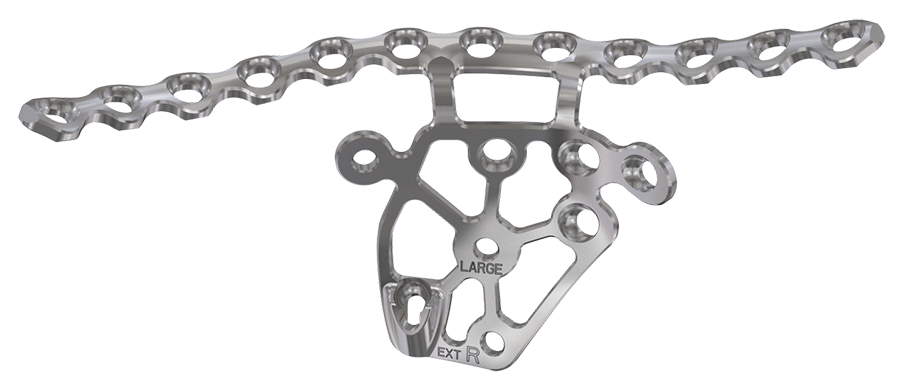
The 3.5 mm intrapelvic acetabular plating system offers four anatomically precontoured plates per side to address fracture and patient variation. For each side these include a small and large sized plate in standard and extended version. Implants are provided in sterile packaging and are manufactured exclusively from stainless steel.
The plate offers superior anatomical fit. An extended version includes an additional posterior screw hole, with the posterior tail of the plate extending lateral to the sacroiliac joint. The plate provides contourable quadrilateral surface extension with improved screw anchorage options in the sciatic buttress, ischium, and posterior column.
Angulated screw holes allow for an engagement with the protection sleeve used for placing screws. Recesses on the outer contour of the plate allow for flexibility when placing additional screws outside the plate.
References
- Rinne PP, Laitinen MK, Huttenen T, et al. The incidence and trauma mechanisms of acetabular fractures: a nationwide study in Finland between 1997 and 2014. Injury. 2017 Oct;48(10):2157–2161.
- United Nations, Department of Economic and Social Affairs, Population Division. In: World Population Prospects: The 2017 Revision, Key Findings and Advance Tables. Working Paper No. ESA/P/WP/248. New York: United Nations; 2017.
Reduction of the system's complexity has also been addressed with new and updated instruments and retractors, designed to ease exposure, plate, and screw insertion.
Specific radiolucent retractors were designed for superior pubic ramus, iliac fossa, and quadrilateral surface. Each offers a method to help maintain retractor position without interfering with the placement of the acetabular plate. The QS retractor includes a channel for the attachment of an optional light strip.
New reduction instruments provide a longer working length compared with standard low-profile pelvic sets. Pointed reduction forceps are offered in right, left, and straight versions. Ball-spiked reduction forceps and ball-spiked pushers can be used with optional spiked discs, if necessary.
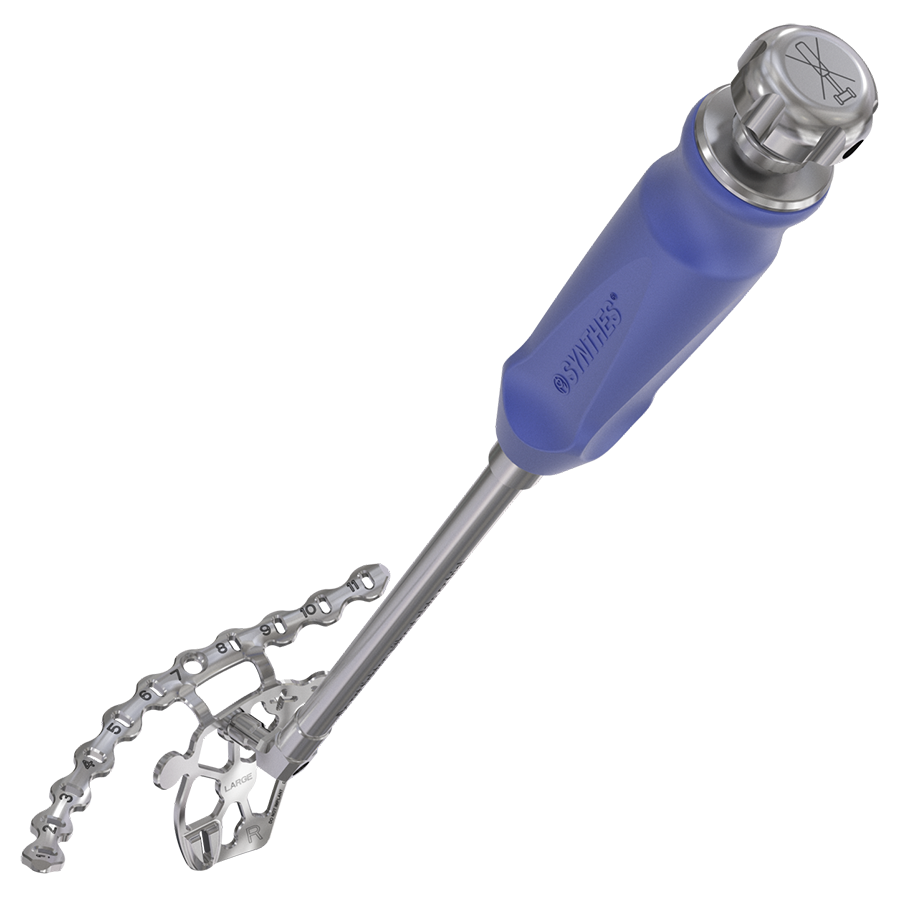
A plateholder provides a stable connection to the plate or sizing trials, which are included in small and standard size and for the left and right sides. They ensure that the most suitable plate size is identified to fit the patient anatomy and the fracture pattern. For additional adjustments, ex-situ and in-situ bending instruments allow the surgeon fine-tuning implants to optimize bone-plate contact.
Screw insertion is facilitated by an innovative through-plate screw insertion concept to reduce the potential of soft-tissue exposure to the drill and the risk of losing a screw or its trajectory. Drilling, measuring, and screw insertion is done through one single sleeve. A dedicated measuring device, drill bits, drill guides, and 2.5 hex/T15 screwdrivers support the process of screw insertion.
Intrapelvic Acetabular System
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.