MAXFRAME AUTOSTRUT™
Theodor F. Slongo, J. Spence Reid
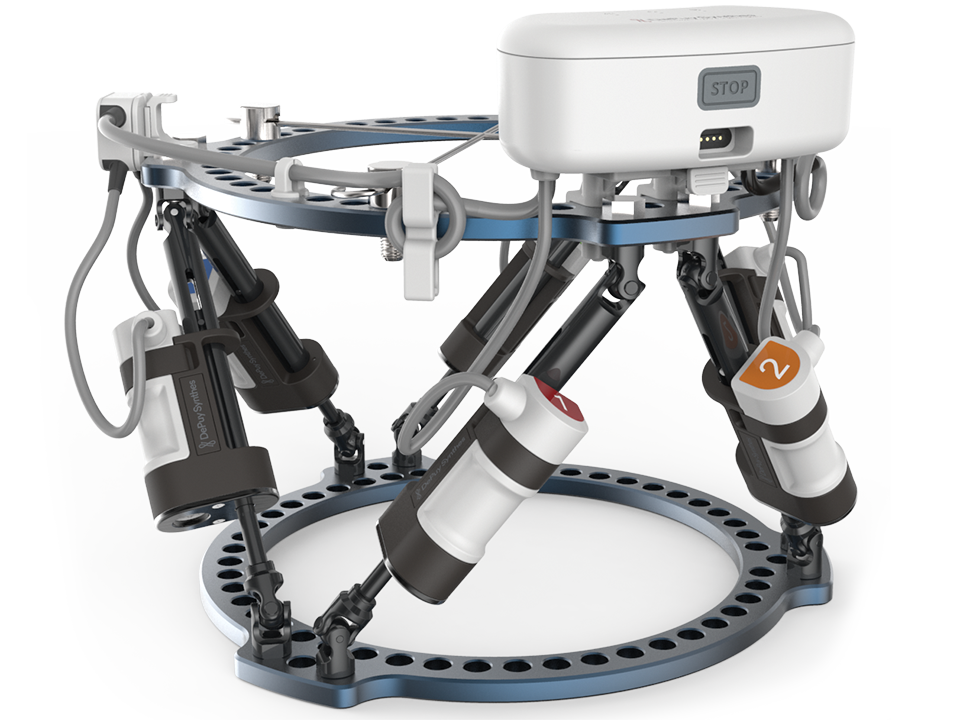
MAXFRAME AUTOSTRUT™ Multi-Axial Correction System is a first-of-its-kind fully automated hexapod ring-fixation system. It provides a solution to the drawbacks of hexapod external ring-fixation treatment by automating the strut adjustment process. Patients no longer need to manipulate the struts, leading to a superior experience throughout the treatment, and reducing the risk of negative clinical outcomes caused by unintended strut adjustments.
It further enables smaller, more frequent actuations, up to 20 times per day. This may be better for the surrounding soft tissue, reduce pain, and improve the quality of the bone regenerate vs. larger and less frequent strut adjustments.
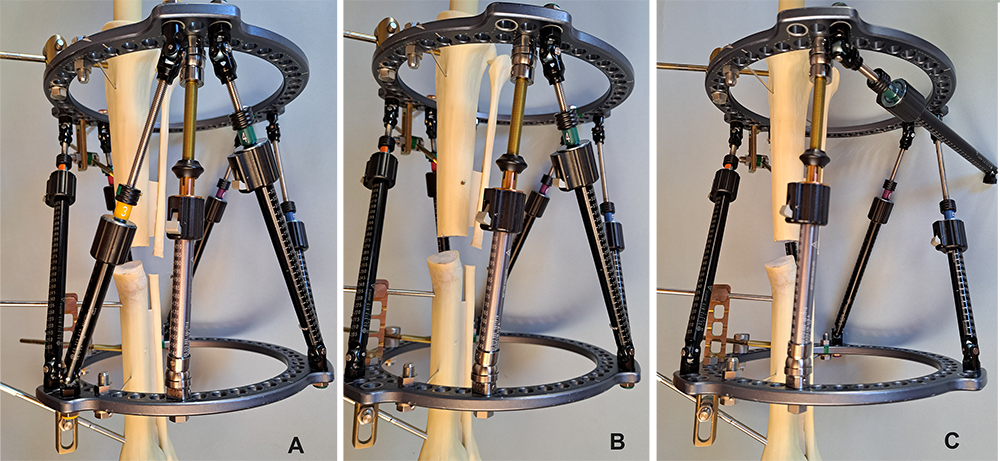
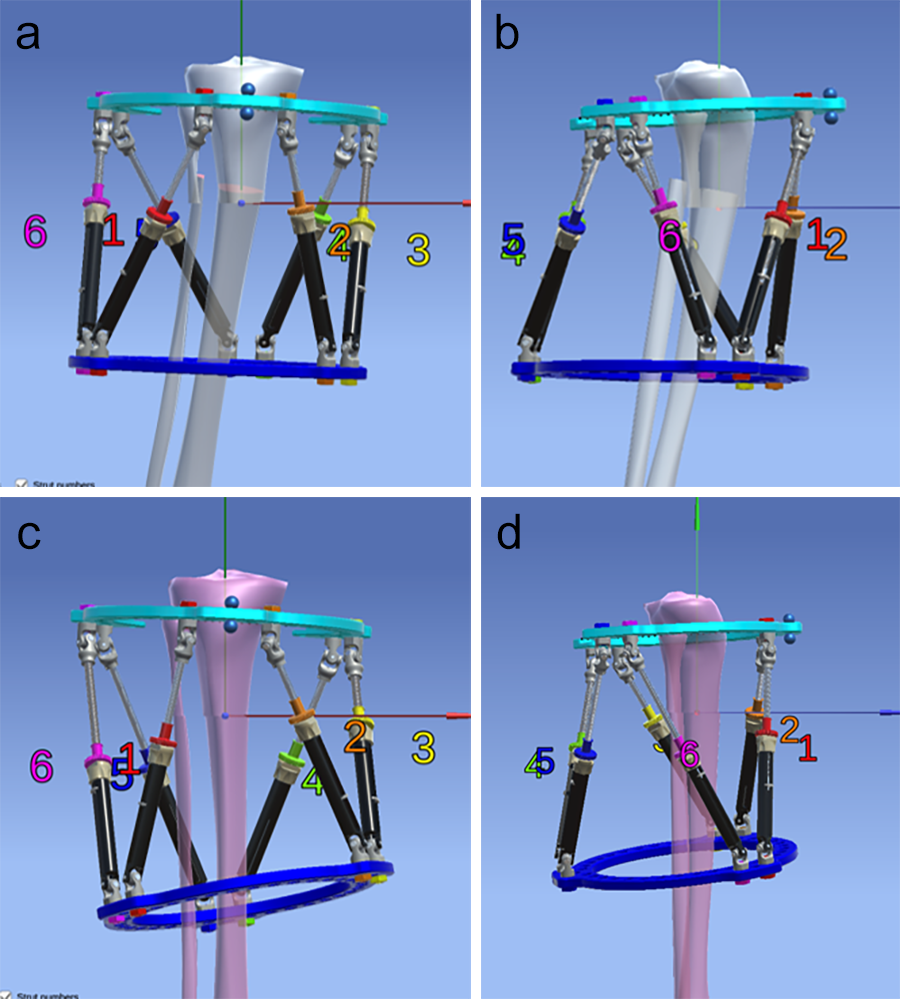
The multiaxial correction system MAXFRAME™ has now firmly established itself as an integral part in the treatment of any bone deformity, be it posttraumatic, infectious, or congenital in origin. The implementation of new hardware components, such as the previously presented linear struts, have again increased the versatility and stability, and made the application simpler. The temporary use of the polyaxial struts is particularly helpful in the case of a strut change, as they guarantee high stability while performing a strut change and are easy to install (Fig 2).
An update was also carried out on the software side, seamlessly compatible with previously created treatment plans and allowing direct transfer to the new software. The current web-based user interface can be accessed on https://www.maxframe3dii.com.
The new software version addresses and integrates the innovative new hardware components MAXFRAME AUTOSTRUT. It enables full automation of strut adjustments through motorized struts and provides a solution to various drawbacks of a traditional hexapod external ring-fixation treatment.
MAXFRAME AUTOSTRUT allows more frequent actuations and higher distraction rates, which may improve callus formation. Simultaneously, it takes into consideration the surrounding soft tissue and nerves, which experience a gentler distraction process when using MAXFRAME AUTOSTRUT vs manually operated struts. Patient discomfort and pain may be also reduced using a larger number of adjustments per day with smaller actuation steps. Currently, up to 20 steps per day are possible. Closed nonunion treatment particularly benefits from this increase in cycles per day while at the same time not increasing demands on manipulations executed by the patient. The system operates completely autonomously and fully automated, not even requiring recharging throughout a treatment.
It is a preference for surgeons and patients not be concerned with strut adjustments, especially during the night. This is achieved with MAXFRAME AUTOSTRUT. Automated strut adjustments may further reduce the risk of negative clinical outcomes caused by unintended or wrong manipulation.
MAXFRAME AUTOSTRUT may additionally allow surgeons to evaluate the progressing stability during the bone-healing phase. Consequently, it is possible to better estimate the load-bearing capacity of the bone at a certain stage of treatment, and potentially allow an earlier removal of the hexapod frame.
The original MAXFRAME hexapod system with the existing software is fully compatible with MAXFRAME AUTOSTRUT.
Specific features and benefits in detail
MAXFRAME AUTOSTRUT System consists of both hardware and software components as follows:
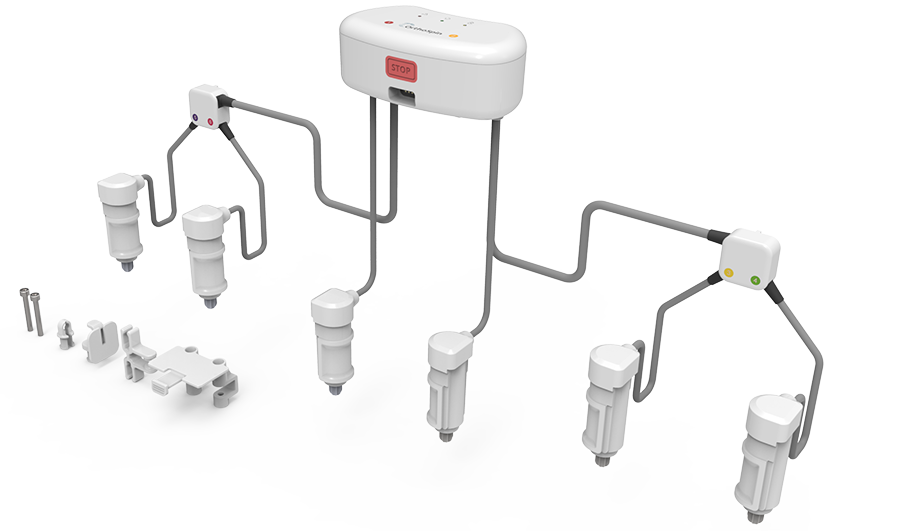
- Hexapod Struts available in three sizes: short, medium, and long (Fig 3)
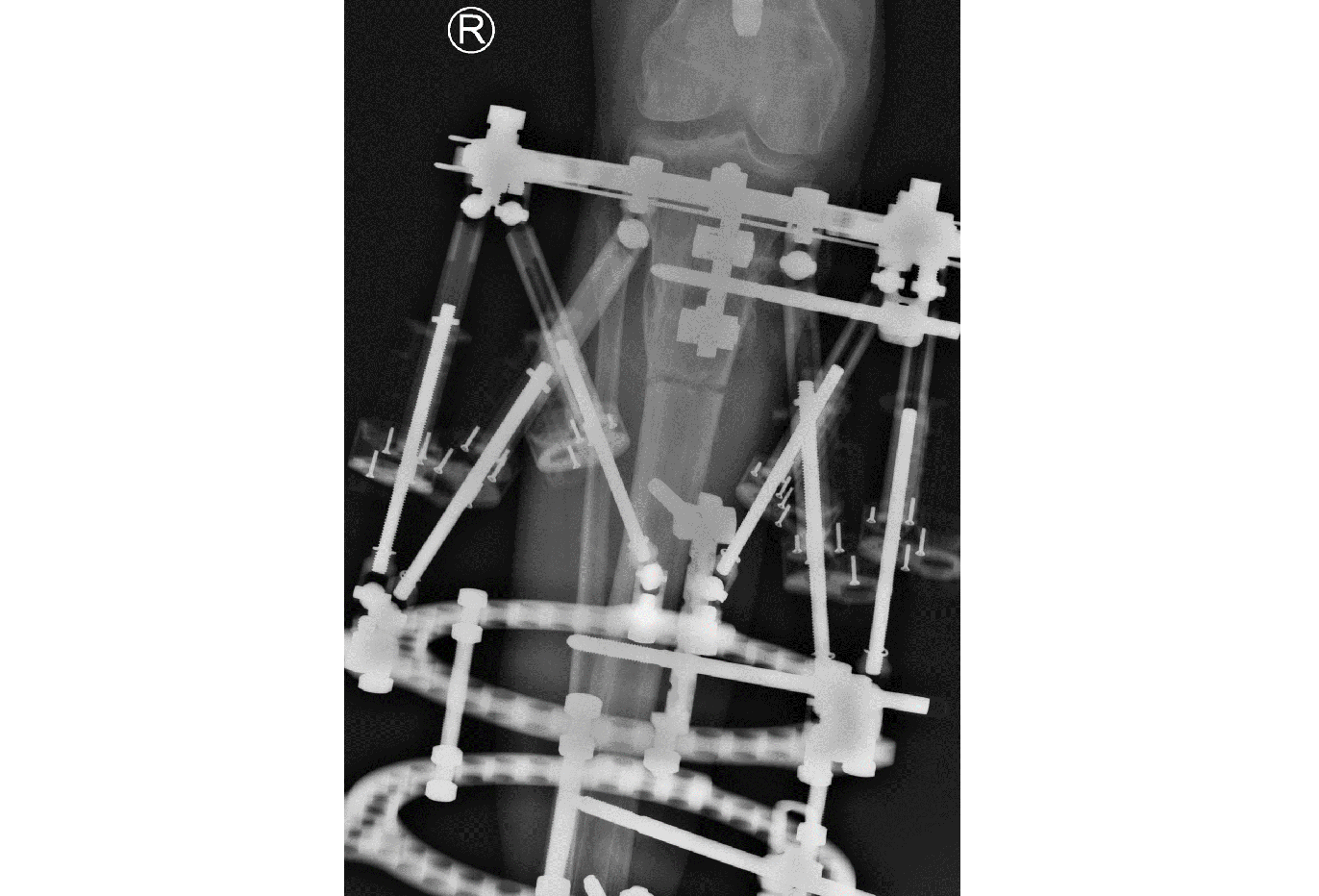
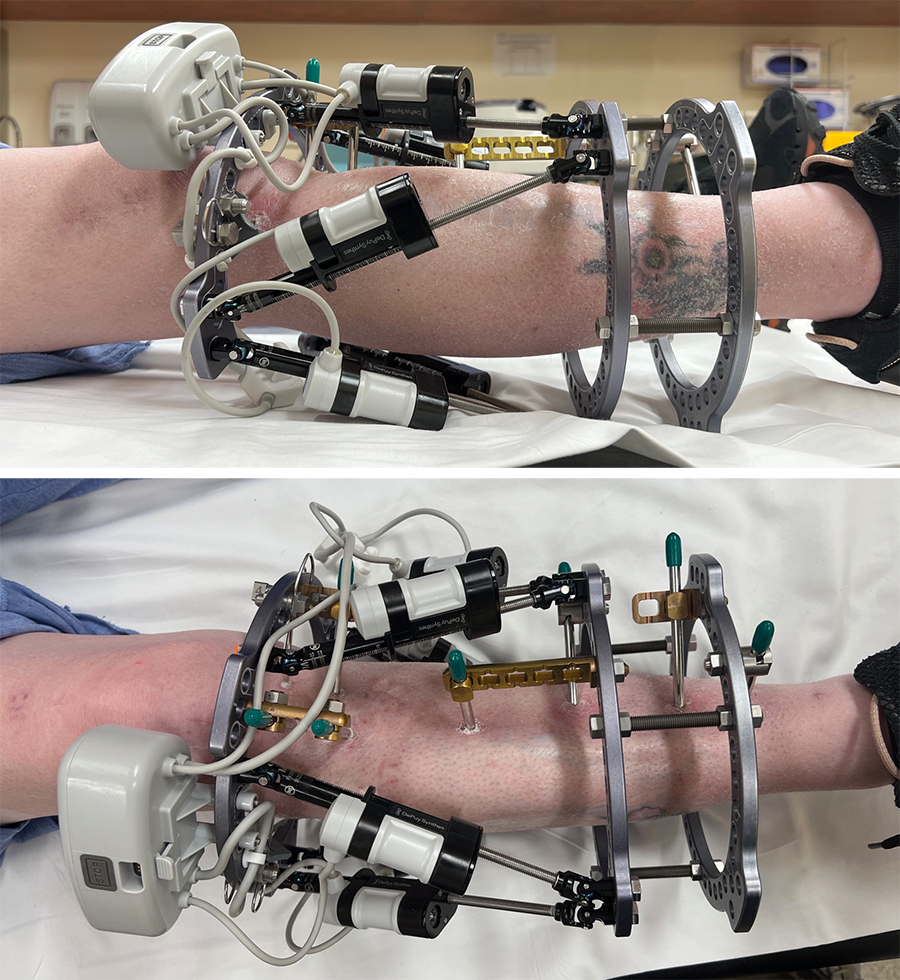
- Automated Hexapod Control System Kit. Control system consists of control unit with a wired connection to six motorized struts (Fig 4)
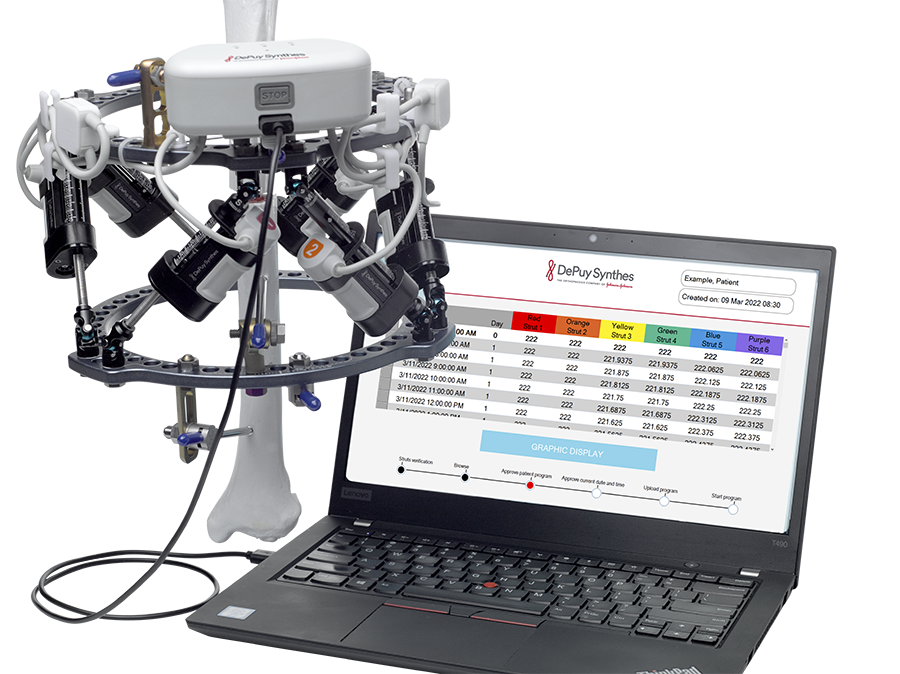
- Software allowing physicians to download the treatment plan to the device, chart patient progress and, if required, adjusting the treatment plan and schedule (Fig 5)
- Accessories
Main indications
A. According to the disease/problem:
- Bone transport
- Lengthening
- Rotational corrections
- Multiaxial corrections
- Nonunions (cycle treatment)
B. Conforming to the patient ability:
- Patients with limited manual ability
- Elderly patients
- Noncompliant or only partially compliant patients
- Children
Operating system and computer hardware
MAXFRAME AUTOSTRUT Software is provided on DPS workstations - the surgeon does not need to understand the operating system. There are some requirements for MAXFRAME 3D II.
For more information and the physician user manual, refer to https://www.orthospin.com/
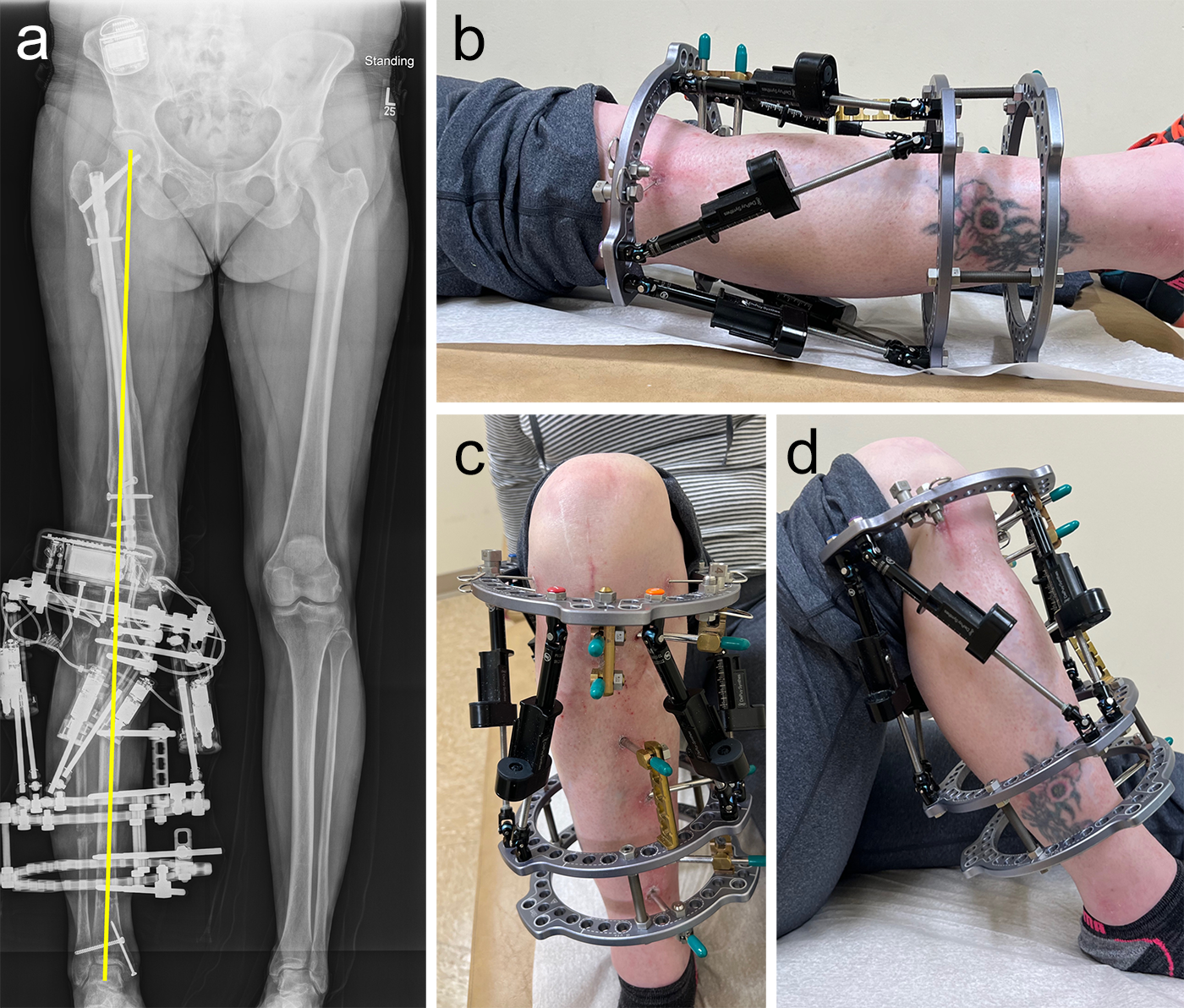
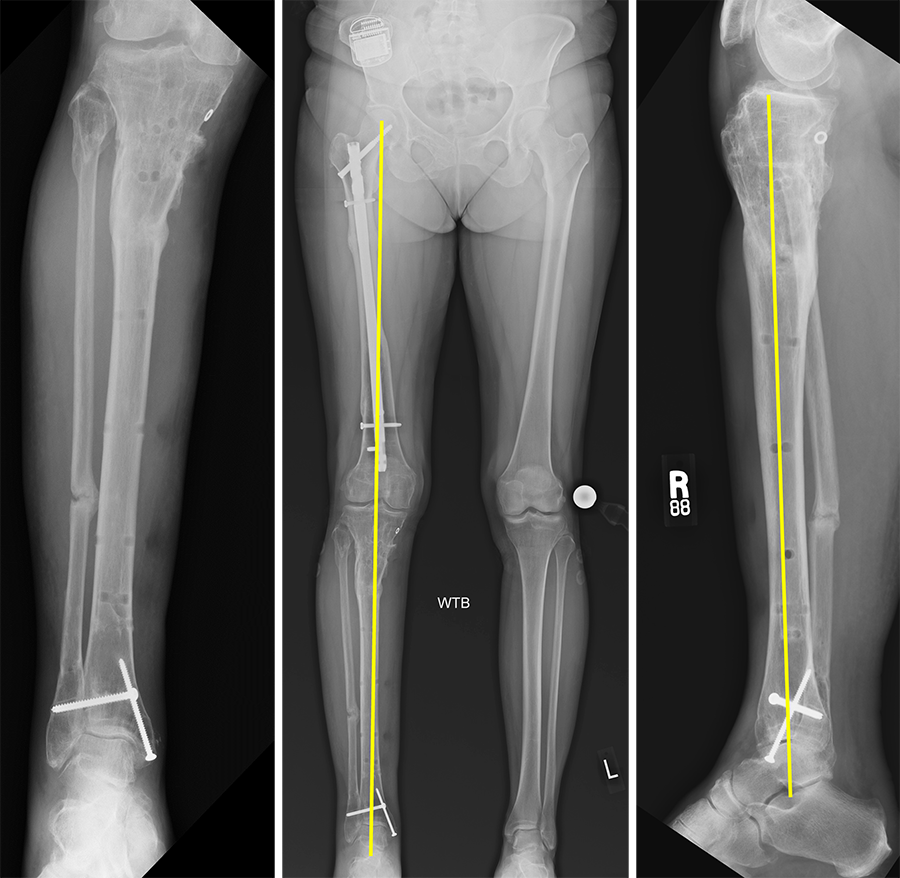
(Case provided by J. Spence Reid, Penn State Health Milton S. Hershey Medical Center, Pennsylvania, USA.)
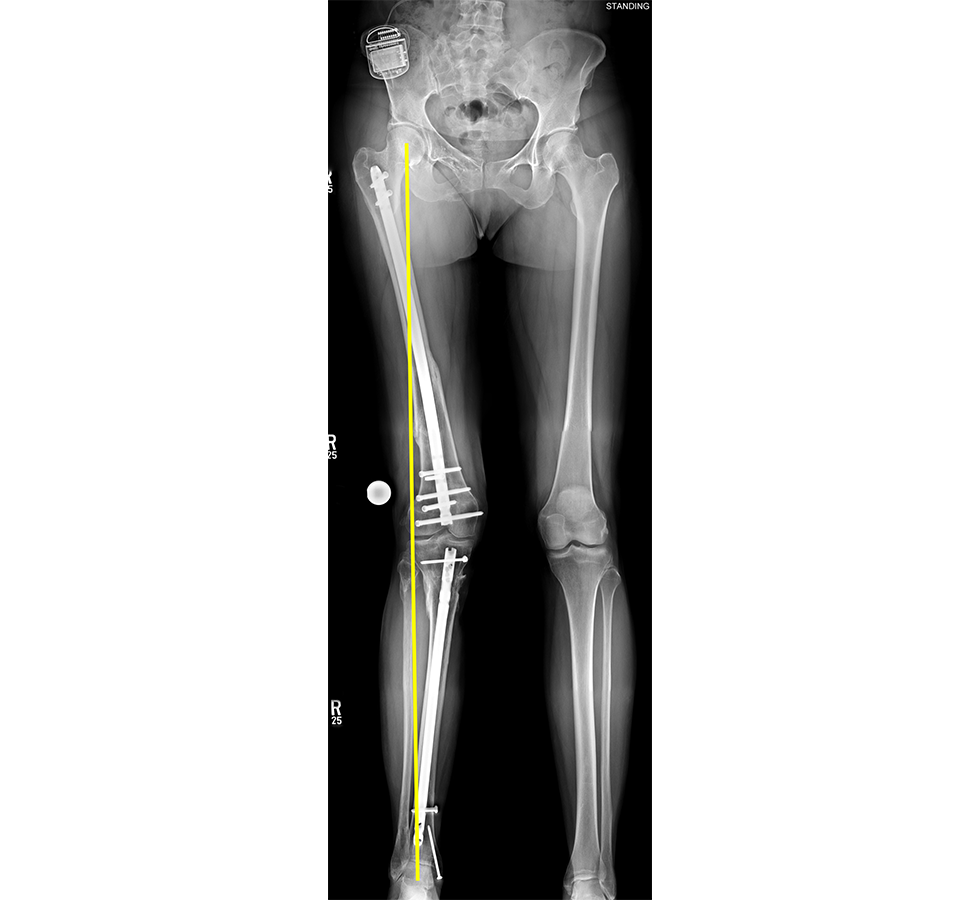
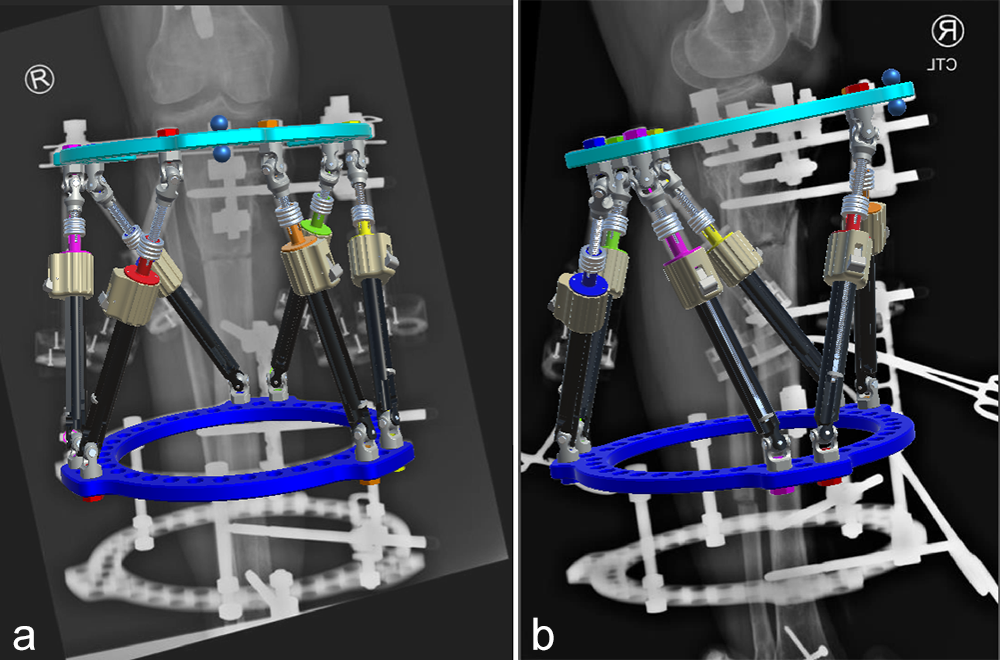
- 47-year-old woman s/p separate femur and tibia trauma
- Valgus knee with external rotation through femur
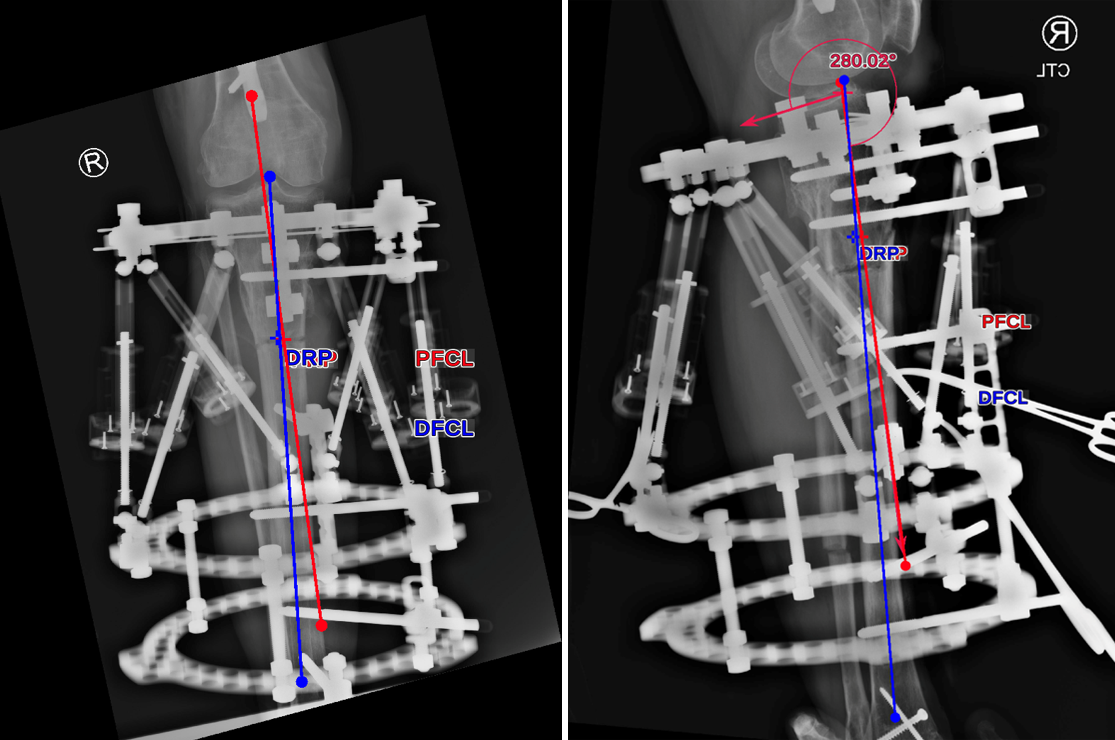
- Deformity analysis revealed that the angular deformity was coming from her proximal tibia.
The treatment plan was to remove the femoral intramedullary (IM) nail, to perform a derotational osteotomy of the femur with an internal saw, and then to refix the femur with a new antegrade IM nail.
The tibial deformity was planned to be managed with a MAXFRAME AUTOSTRUT™.
MAXFRAME AUTOSTRUT™
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.