TRUMATCH™ Graft Cage – Long Bone
Hans-Christoph Pape, Ingo Marzi, Peter Giannoudis, David Hak, Christof Müller, Gerhard Schmidmaier, Martijn Poeze, Brett Crist, Brent Norris
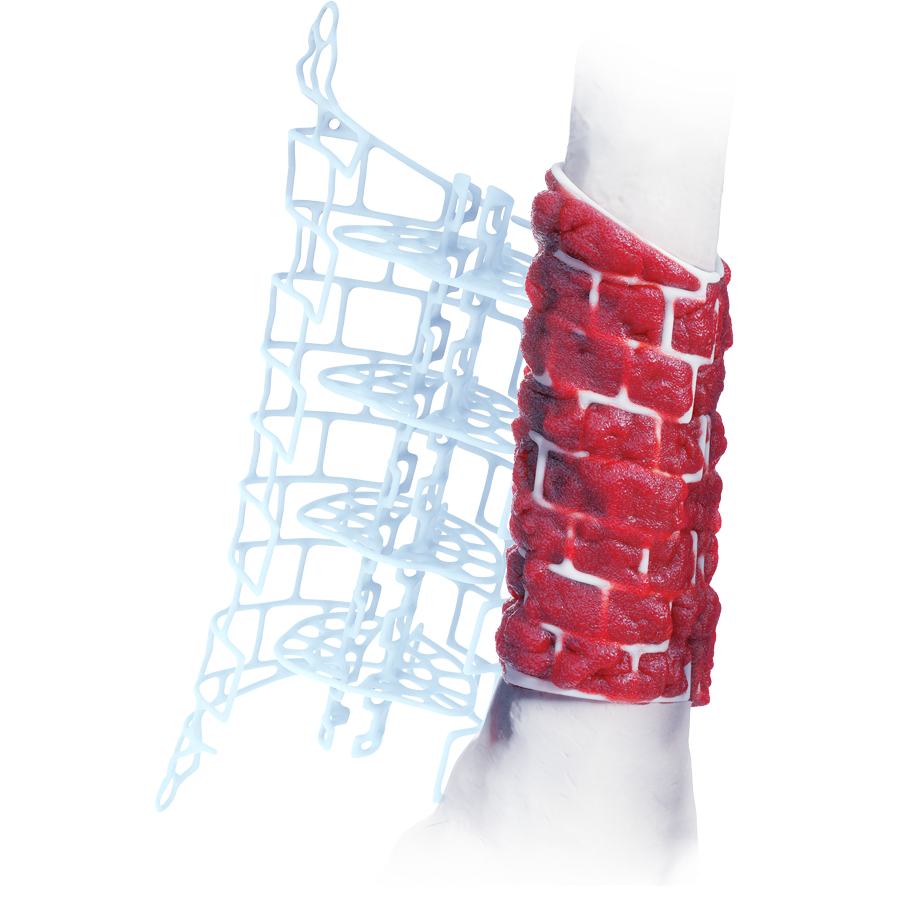
The treatment of segmental bone loss due to trauma or infection remains a challenging clinical problem with variable outcomes. The TRUMATCH™ Graft Cage – Long Bone (Fig 1 ) is an innovative solution allowing improved healing of critical size defects in skeletal long bones (femur, tibia, and humerus).
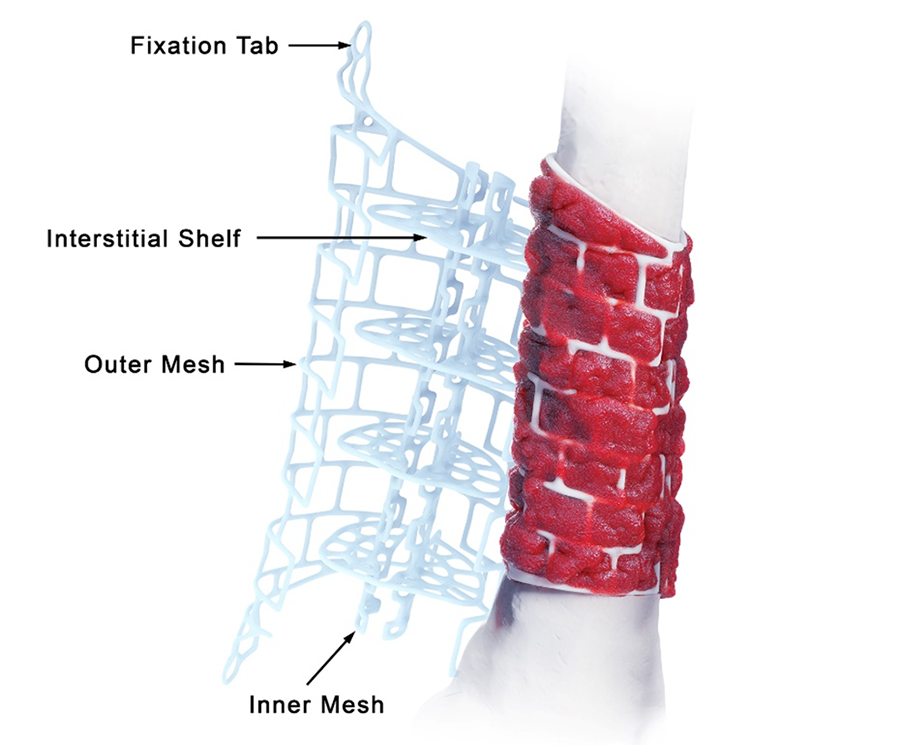
Personalized and bioresorbable, the implant is designed to contain morselized bone graft and can be tailored to the specific anatomy of the defect. The graft cage is intended for use in the second stage of the Masquelet technique in conjunction with rigid fixation and holds bone graft in position for the duration of healing. The cage structure helps prevent the collapse of bone graft, and the mesh design allows vascular ingrowth and nutrient access for tissue remodeling (Fig 2, in details tab).
Clinical problem
Large bone defects are difficult to treat and are associated with a high reintervention rate. High risk of complications such as nonunion, malunion, deep infection, and significant healthcare costs are not uncommon.1 68% of large defects occur in the tibia following trauma, and 22% in the femur.2,3 The rate of nonunions can be as high as 66%, malunions 12%, and donor-site complications (following bone-graft harvesting) as high as 21%.4 Surgical challenges in managing such large bone defects include the eradication of infection, achieving bone union, and achieving stability/fixation. A further complicating factor is the anatomy of bone defects, which are highly variable. The defect can range from 2.5 cm to 30 cm, with a cross-sectional shape dependent on the bone and the location of the defect, and end geometry ranging from angled or jagged in trauma cases to parallel and smooth in oncology resections. 2,3
State of the art
Whilst there is no standard treatment protocol for large bone defects, the two most common techniques are the induced membrane technique (Masquelet technique) and distraction osteogenesis by bone transport. The Masquelet technique is a relatively recent treatment option consisting of two stages. In the first stage a biological membrane is formed around a cement spacer which is inserted in the bone defect. In the second stage, the spacer is carefully removed and the membrane filled with bone graft.5 The induced membrane functions to contain the graft and stimulate bone-graft remodeling. Distraction osteogenesis or internal bone transport is based on the regeneration of bone by gradually separating bone segments with fixation devices (eg, external fixator, distraction nail) creating a gap that stimulates new bone formation. The treatment choice depends on the location and size of the defect, patient physiology, and surgeon skill set.
The Masquelet technique offers several advantages over distraction osteogenesis:
> The Masquelet technique creates a favorable biological environment for bone regeneration. The induced membrane formed during the initial stage of the procedure promotes the development of new blood vessels and supports the integration of the bone-graft material. This environment encourages the formation of new bone and facilitates healing.
> Despite being a two-stage procedure, patient treatment with the Masquelet technique is generally simpler and less time-consuming compared to distraction osteogenesis.
> The Masquelet technique avoids the following risks associated with distraction osteogenesis: pin-track infections, device-related issues, and the need for hardware removal after bone consolidation.
> The Masquelet technique can address limitations of distraction osteogenesis regarding the maximum defect length and defect shape amenable to treatment.
> The fixation devices and materials required for the Masquelet technique can be less expensive compared to more sophisticated bone transport devices (eg, for motorized distraction).
> There is no patient intervention required for the Masquelet technique, whereas patient compliance is needed to initiate or perform the individual distraction steps in distraction osteogenesis.
However, there are also challenges associated with the Masquelet technique, including the availability of sufficient graft material, adequate containment of bone graft, the mechanical stability of the graft in larger defects, and risk of introducing or eradicating deep infection. Some of these challenges are addressed in the design features of the graft cage.
Clinical solution
The TRUMATCH™ Graft Cage – Long Bone (Fig 2) is a 3D-printed, patient-specific, resorbable implant. The cage material comprises 96% polycaprolactone and 4% hydroxyapatite which resorbs slowly over 2–4 years. Thus, the graft cage provides internal structural support to the graft material for the duration of healing, while providing large pathways through the mesh openings for nutrient flow and revascularization from the surrounding tissues. The hydroxyapatite/calcium phosphate coating is osteoconductive, promoting mineralization at the surface of the implant and eventual conversion to bone.
Design features
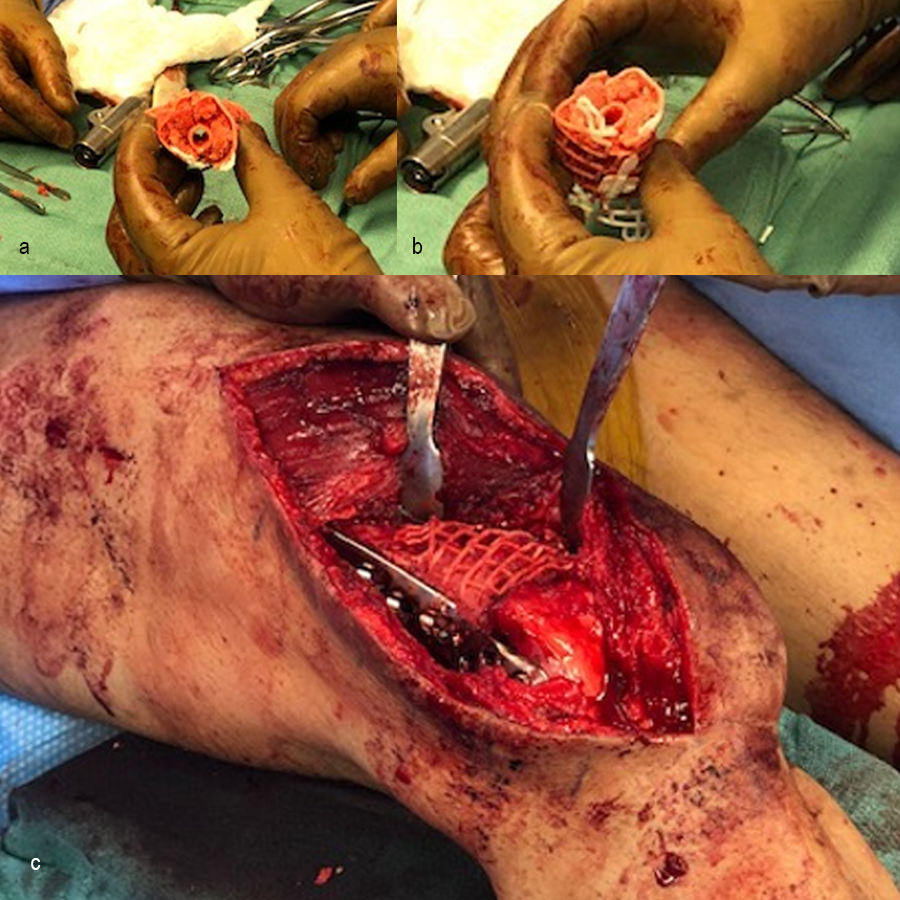
The outer mesh of the graft cage (Fig 2) can be produced to approximate the cortical surface of the missing bone within the defect, thereby mimicking the previous bone shape. The two halves of the outer mesh are hinged such that they can be opened to facilitate graft packing. Large windows in the outer mesh allow exposure of the packed bone graft to the surrounding soft tissues for vascular ingrowth. The tube-in-tube design supports vascular ingrowth both circumferentially and through the intramedullary canal.
The inner mesh is made to approximate either the intramedullary canal or an intramedullary nail. The smaller window size in the inner mesh prevents graft subsidence into the intramedullary region of the implant while still allowing nutrient inflow. The tubular graft construct reduces the amount of graft needed compared to filling the entire defect. The inner mesh is hinged to allow the entire implant to open for ease of insertion over an intramedullary nail. The interstitial shelves are porous, are spaced equally along the length of the implant, and provide vertical support throughout the graft.
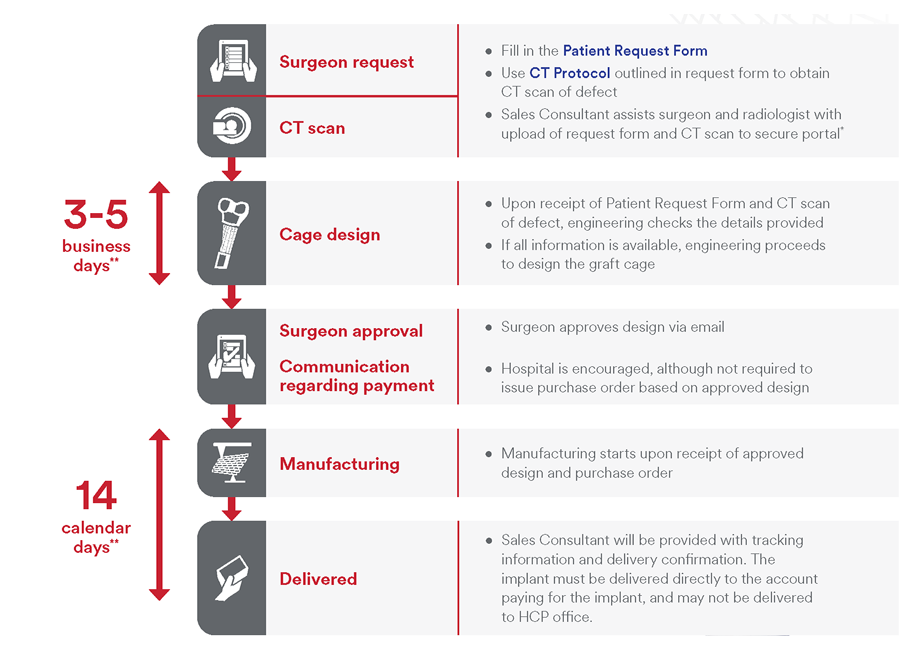
Preoperative workflow
The process begins with a CT scan of the defect being generated at the hospital (Fig 3). Computed tomographic data is sent to J&J MedTech where proprietary software segments the CT data and customizes the implant. Following clinician approval of the final design, the graft cage is then 3D printed and given an osteoconductive coating. The implant is packaged, terminally sterilized via ethylene oxide, and shipped to the patient.
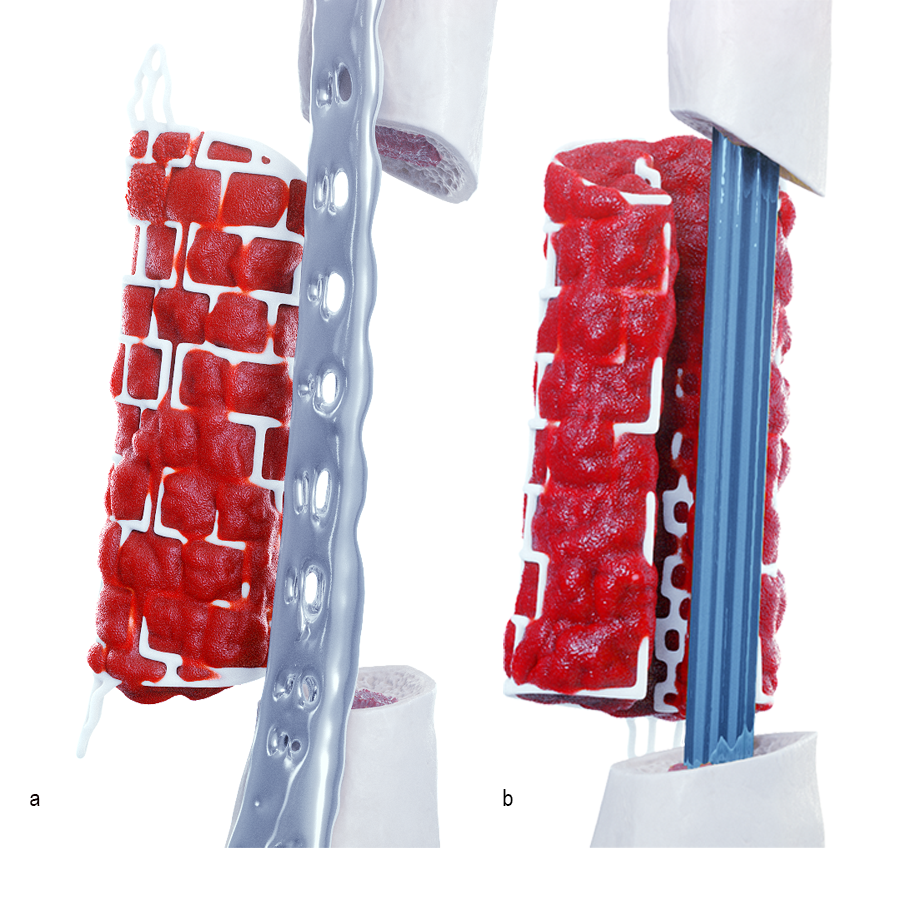
Operative workflow
In the operating room, rigid fixation (an orthopedic plate, external fixation, or an intramedullary nail) is applied (see Clinical Case tab for details). Bone graft is then harvested, either from the iliac crest or via the reamer-irrigator-aspirator (RIA). The outer mesh of the graft cage is opened for packing graft inside, then the cage is implanted. An inner mesh opening is provided for fitting around an intramedullary nail during this step. The graft cage offers benefits over other technologies in that it contains shelves to support the graft, as well as patient-specific contouring better suited for complex anatomies.
Value proposition/benefits of the TRUMATCH™ Graft Cage – Long Bone
The graft cage retains bone-graft material at the desired location, which has the potential to increase bone-union rate, decrease the time-to-union, and reduce the requirement for revision surgeries.6 Additionally, the graft cage may drive cost savings for the provider and payer by reducing complications such as nonunions, reducing the number of surgeries, and reducing allograft usage. Overall, both patient satisfaction and procedure efficiency may be improved.
Nonclinical testing and validation
In a study comparing the healing of ovine segmental tibial defects treated with Orthomesh or the TRUMATCH™ Graft Cage – Long Bone, the graft cage group had more robust and advanced bone healing after 18 weeks. Animals treated with the graft cage had greater (by 55%) final bone volume, and a faster transition from woven to dense bone.7,8
Although there was no significant difference in bone union score, the graft cage group showed significantly higher torsional strength, lower pain scores, suggestive evidence of increased ingrowth/integration of tissue, and favorable biocompatibility.7,8
Intended use (United States only)
The graft cage is intended to be used in the treatment of large, segmental, nonarticular bone voids or surgically created resections of the humerus, femur, or tibia in conjunction with traditional, rigid fixation. The TRUMATCH™ Graft Cage – Long Bone is intended as an implantable structure that can support grafting materials in these procedures.
Indications (United States only)
> Maintaining the relative position of bony tissue such as bone grafts, bone-graft substitutes, or bone fragments from comminuted fractures within nonarticular bone voids or surgical resections of the humerus, femur, or tibia
> Load-bearing applications, only when used with traditional, rigid fixation
> Skeletally mature adults and adolescents
> Skeletally immature adolescents, as long as the device is not used across open physes
Contraindications (United States only)
> Bone voids or surgical resections that include articular surfaces
> Bone voids or surgical resections that use the device across open physes
> Load-bearing applications where no traditional, rigid fixation is present
> Use in the spine
> Patients with a compromised ability for bone healing (eg, active infections, poor bone quality, insufficient blood supply, etc)
> Use in patients requiring acute/emergent treatment due to the time requirements to personalize, manufacture, and deliver the device
For intended use, indications, and contraindications in regions outside the United States, please consult the appropriate local product labeling.
References
- Norris BL, Vanderkarr M, Sparks C, et al. Treatments, cost and healthcare utilization of patients with segmental bone defects. Injury. 2021 Oct;52(10):2935–2940.
- Molina CS, Stinner DJ, Obremskey WT. Treatment of Traumatic Segmental Long-Bone Defects: A Critical Analysis Review. JBJS Rev. 2014 Apr 1;2(4):e1.
- Olson Market Research. Interviews conducted with 71 surgeons from US, UK, Germany, Italy, China, Japan, Australia, Brazil, and Mexico. 2015.
- Vanderkarr M, Sparks C, Wolf S, et al. Patient Characteristics and Healthcare Utilization Following Comminuted Type III Fractures. Submitted to ISPOR Europe 2019.
- El-Hadidi TT, Soliman HM, Farouk HA, et al. Staged bone grafting for the management of segmental long bone defects caused by trauma or infection using induced-membrane technique. Acta Orthop Belg. 2018 Dec;84(4):384–396.
- DePuy Synthes. DePuy Synthes Design Print for TRUMATCH Graft Cage - Long Bone. December 2, 2019. Windchill Document No. SD900_500S.
- DePuy Synthes. Design Verification Report - Ovine tibial Critical Defect Model, Pre-clinical Study - PSC-CORL 008. May 1, 2018. Windchill Document No. 0000273020.
- DePuy Synthes. Longitudinal Tibial Bone Healing Assessment. November 28, 2017. Windchill Document No. 0000271732.
- Morwood MP, Streufert BD, Bauer A, et al. Intramedullary Nails Yield Superior Results Compared With Plate Fixation When Using the Masquelet Technique in the Femur and Tibia. J Orthop Trauma. 2019 Nov;33(11):547–552.
Case 1—Open right supracondylar intracondylar distal femoral fracture following motor vehicle accident
(Case kindly provided by Brent Norris, Orthopedic and Trauma Services of Oklahoma, USA)
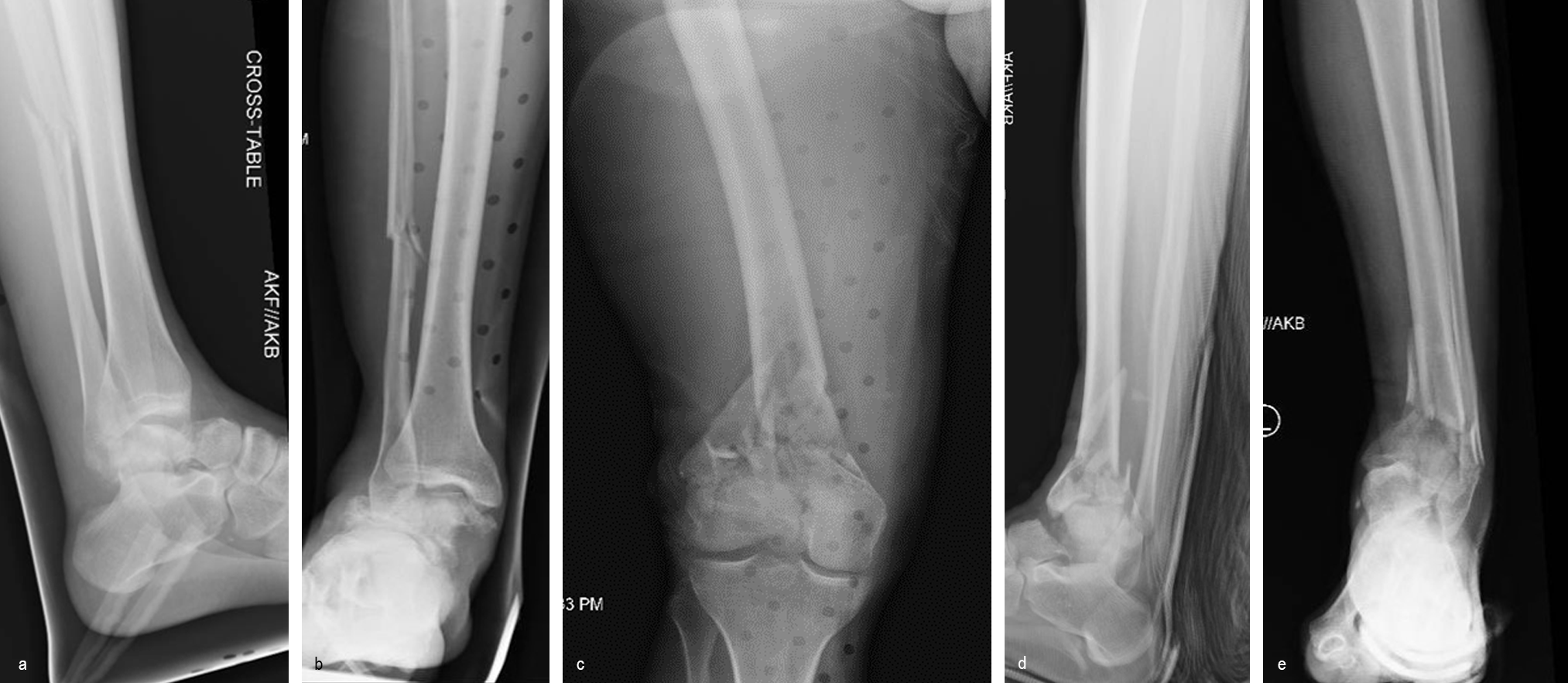
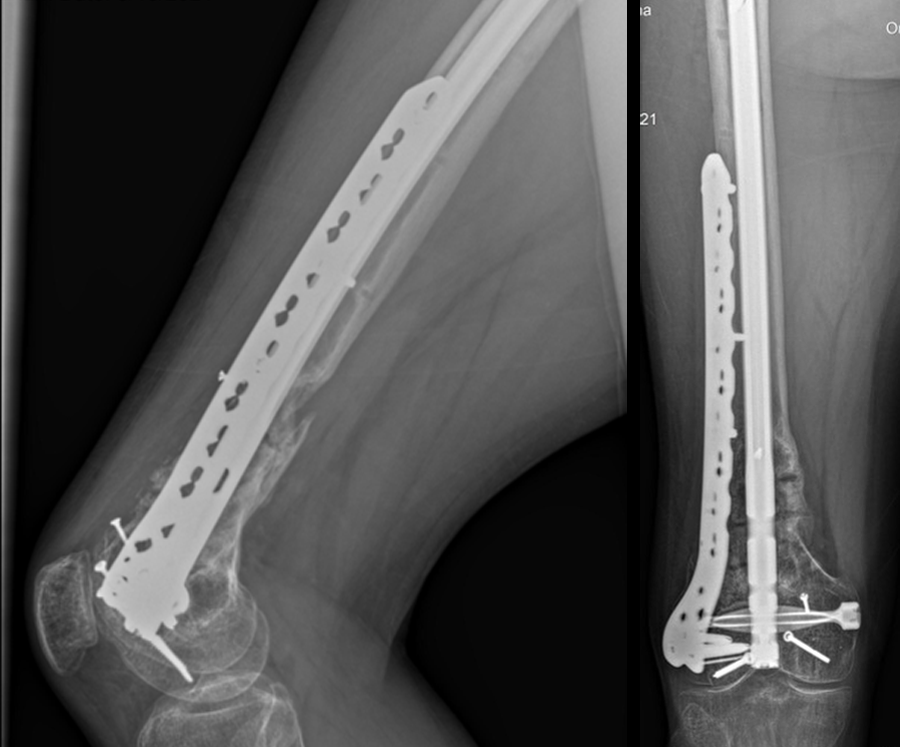
A 34-year-old man was involved in a motor vehicle accident in December 2019 and sustained these injuries (Fig 5):
> Left-sided rib fractures with lung contusion
> Open right supracondylar intracondylar distal femoral fracture with possible vascular injury (limb-threatening injury)
> Closed right bimalleolar ankle fracture
> Closed right talar fracture
> Open left plafond fracture
> Open left talar fracture dislocation
> Open left elbow joint
He had no major previous medical history and worked in the furniture delivery business.
The day following admission, the patient underwent these procedures in the operating room (Fig 6):
> Washout of open injuries right side, including femur and ankle
> Open reduction of the talar injury
> Spanning external fixator of the femur and ankle
> External fixation of the left plafond/talus
At 3 days postoperative, the patient underwent a repeat washout of the right femur and right ankle. The lung injury was still recovering so no definitive fixation was performed at this time.
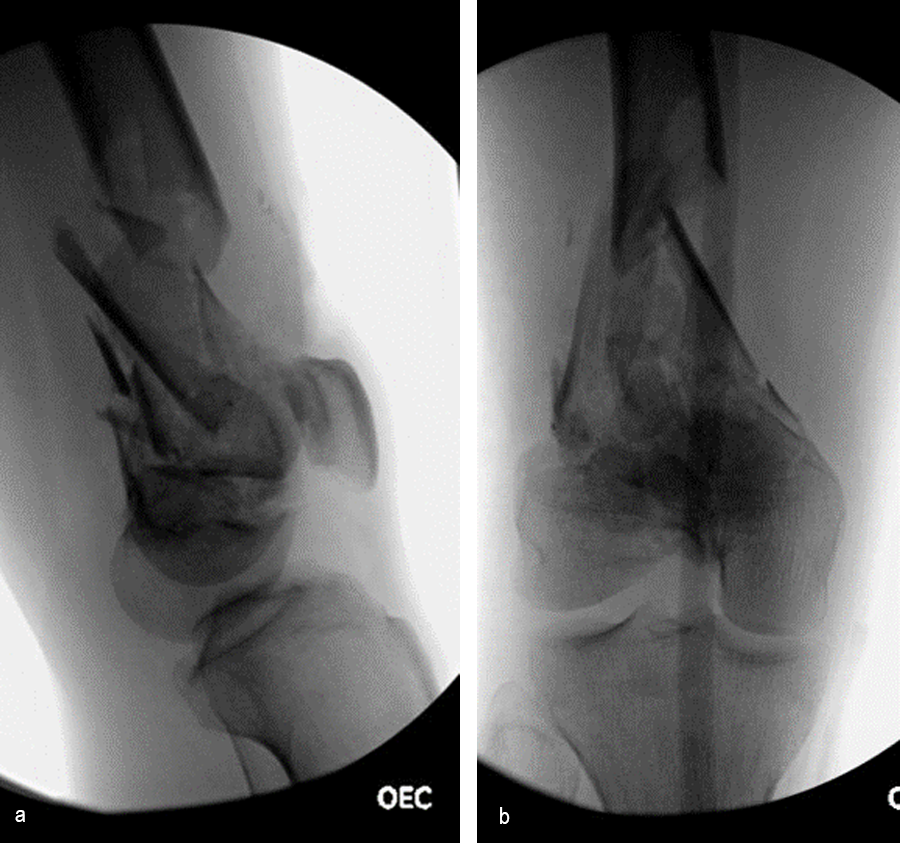
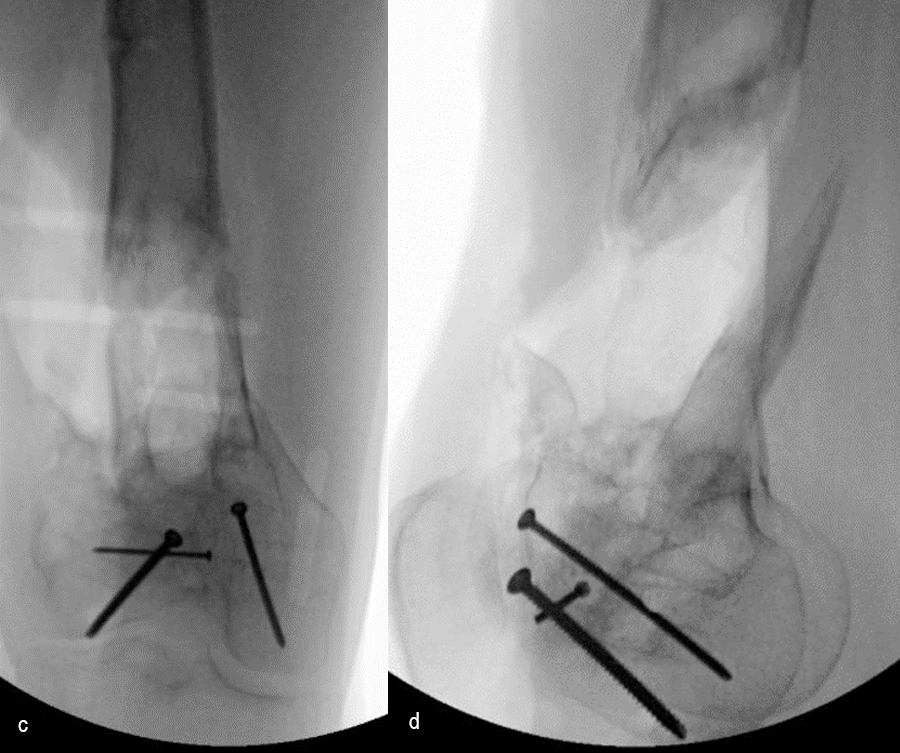
At 5 days post-initial surgery, the lung injury was improved, so the patient underwent open reduction and internal fixation (ORIF) of the right distal femur with resection of devitalized bone and cement spacer placement (Fig 7). Definitive fixation of the other fractures (ankle and ribs) was undertaken over time.
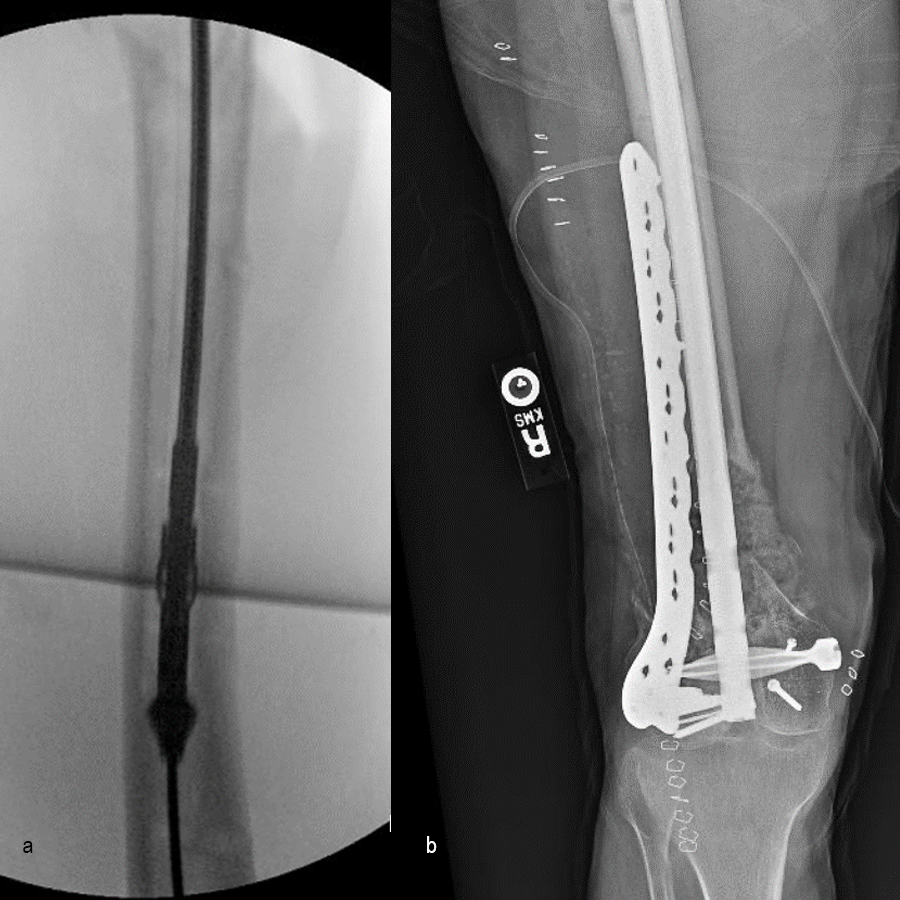
Following the advent of Covid-19 in early 2020, the patient was lost to follow-up for almost one year and was eventually seen again in December 2020 (Fig 8). At this follow-up, a CT scan was planned for the right distal femur and a graft cage ordered. Surgery was scheduled for early 2021.
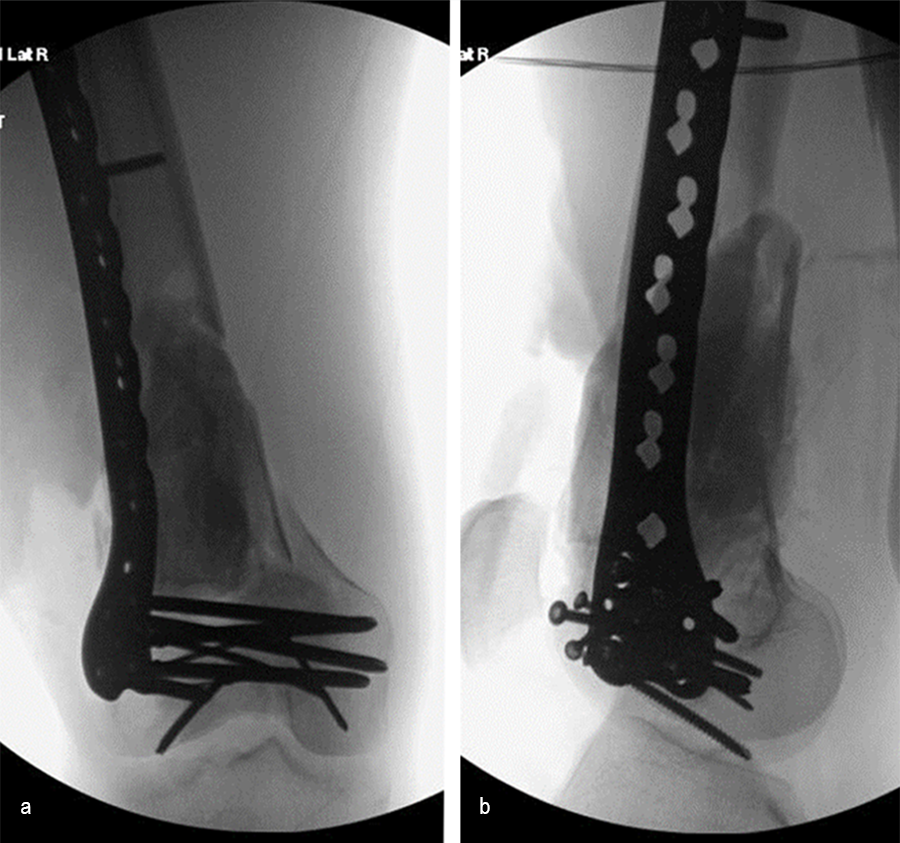
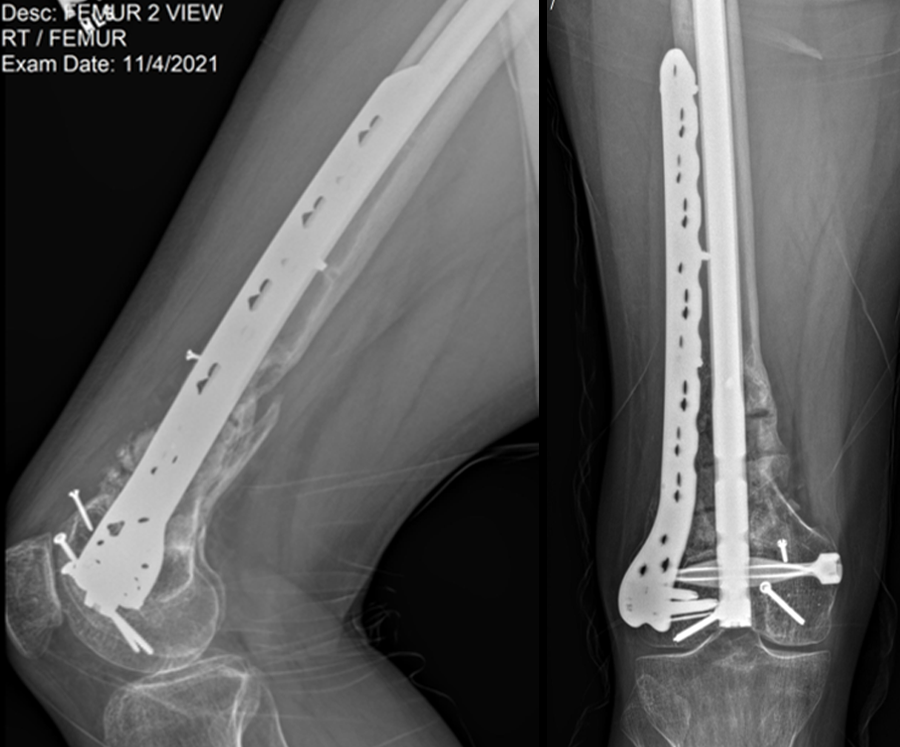
At the 3-week follow-up (post-graft cage, Fig 12), the patient’s wounds were healthy. He had a range of motion (ROM) of 0–80 in his right knee. He was allowed weight bearing as tolerated (WBAT) with crutches.
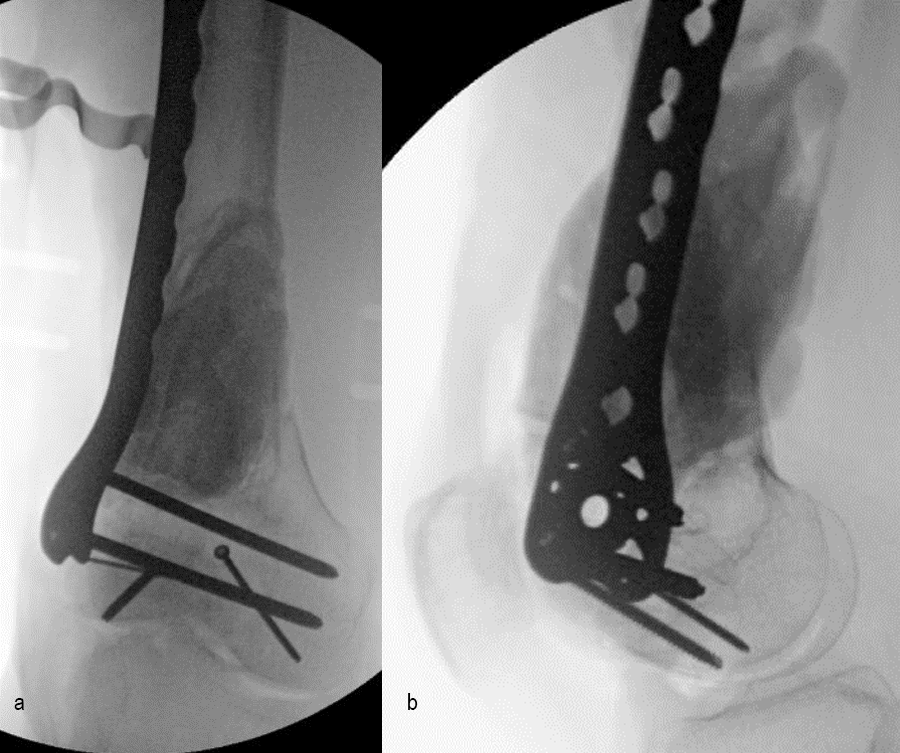
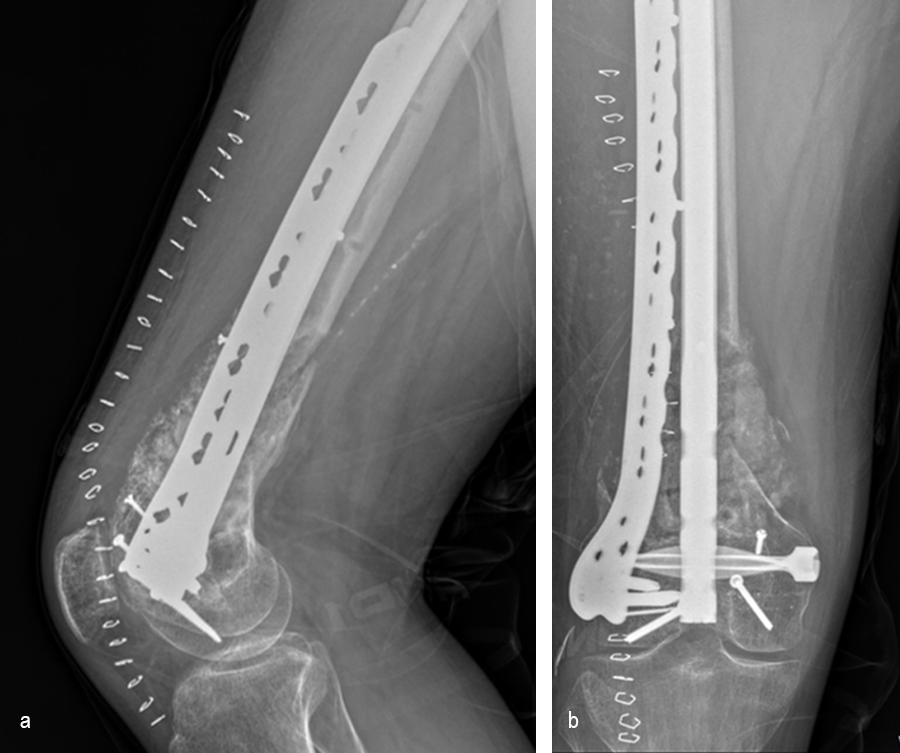
At the 3-month follow-up (Fig 13), the patient had a ROM of 0–120 in his right knee. His quadricep muscles had significantly recovered and he was able to WBAT with support from a cane.
At the 6-month follow-up (Fig 14), the patient was able to WBAT on the right lower extremity. He had some ankle pain and a ROM of 0–125 in his right knee. The patient was able to walk without any assistance device.
At the 9-month follow-up (Fig 15), the patient reported nominal knee pain, but more pain in his ankles. The patient had returned to work. He was not undertaking any heavy lifting but was driving the truck and supervising the team. Overall, the patient was happy with the current outcome of surgery.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.