VA-LCP Clavicle 2.7 System
Simon Lambert, Stefaan Nijs, Martin Jaeger, Harry Hoyen, Chunyan Jiang
The Variable Angle (VA) LCP Clavicle Plate 2.7 System is the next generation of internal fixation for the clavicle designed to treat medial, lateral, and shaft fractures (Fig 1). The system was created in response to clinical challenges in the current treatment of fractures of the clavicle and is available in both stainless steel and titanium alloy.
One of the most common complications when treating clavicular fractures operatively is the need for hardware removal due to irritation caused by prominent plates. The VA-LCP Clavicle Plate 2.7 System was subsequently designed to treat lateral, shaft, and medial fractures in different size clavicles for patients of small, medium, and large stature. Based on extensive analysis of 15 anatomical parameters on more than 600 clavicle CT scans, the shapes of the VA-LCP Clavicle Plate 2.7 are designed to match the bow and contour of the clavicle for low construct prominence and enhanced plate-to-bone fit compared with other systems.
As an addition to the VA-LCP Clavicle Plate 2.7 System, the VA-LCP Clavicle Hook Plate System 2.7 was launched in August 2021 (Fig 2).
As previously emphasized, the most significant clinical challenge associated with clavicle plating is the high reoperation rate. Poor plate fit and high construct prominence in clavicular fracture fixation causes tissue irritation that can result in patient pain, discomfort, and additional surgery to remove hardware. Specific clinical challenges associated with clavicle hook plates are hook impingement and acromial osteolysis.
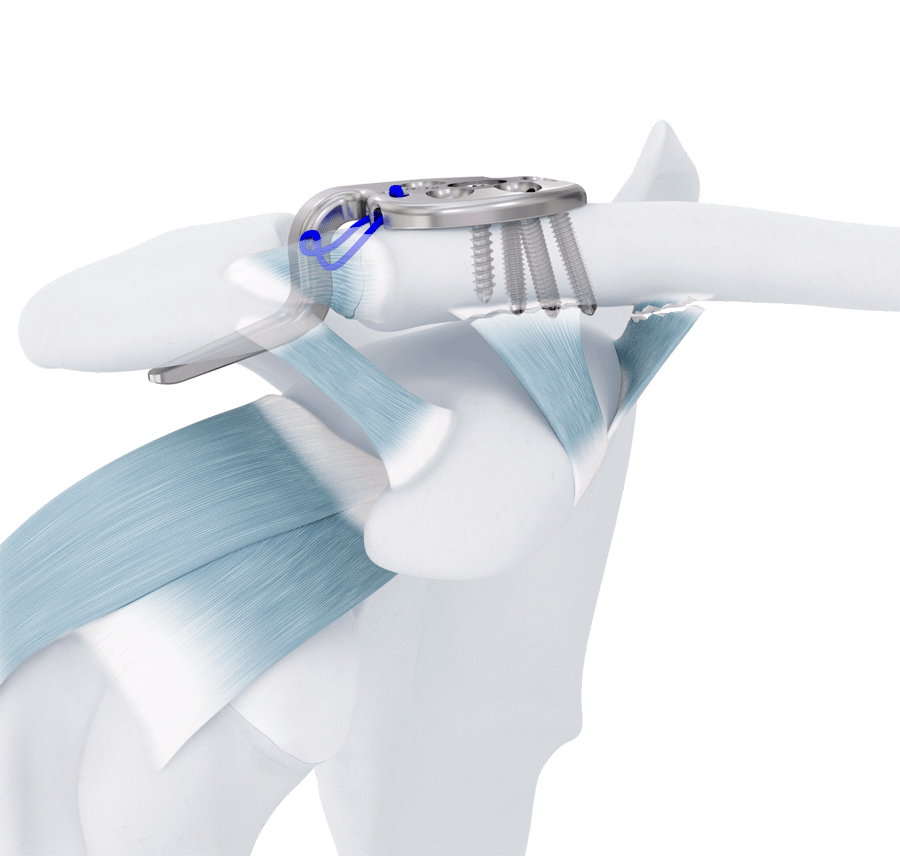
The VA-LCP Clavicle Hook Plate 2.7 System provides a solution for both lateral clavicular fractures with associated acromioclavicular ligament and coracoclavicular ligament injuries and ligamentous injuries of the acromioclavicular joint. Higher grade acromioclavicular joint dislocations require surgical fixation. One option for surgical treatment is the hook plate although its use has historically been associated with pain and impingement causing some patients to request implant removal before complete healing. The VA-LCP Clavicle Hook Plate 2.7 System has enhanced hook geometry designed to reduce pin-point contact of the hook on the underside of the acromion (3D morphometric analysis of the acromioclavicular joint—implications for surgical treatment using subacromial support, unpublished data).
VA-LCP Clavicle Plate 2.7 System
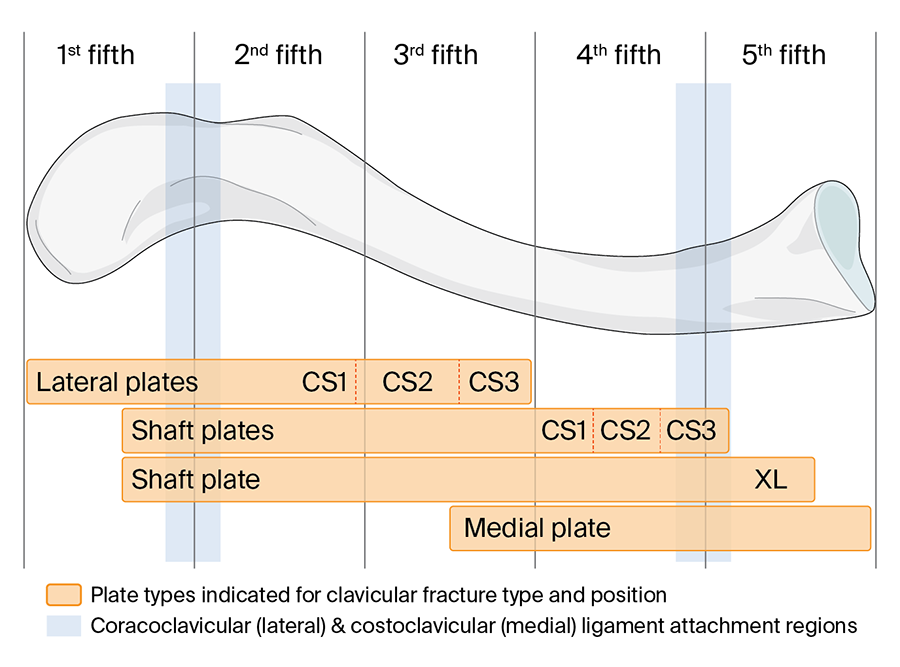
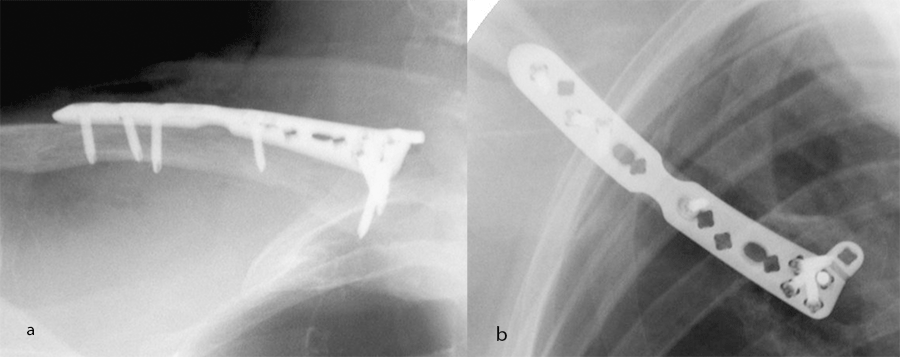
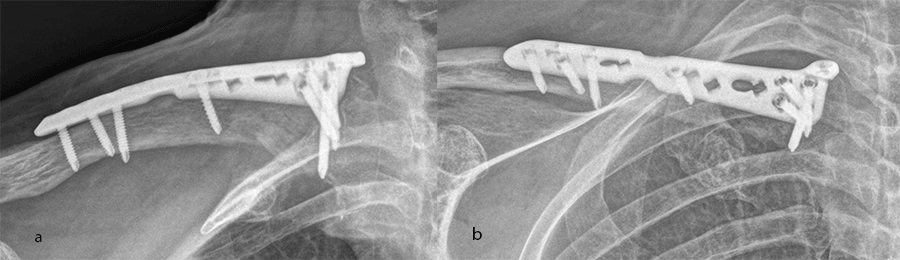
The VA-LCP Clavicle Plate 2.7 System comprises a selection of plates to address fractures in specific segmental regions of the clavicle (Fig 3). Lateral and shaft plates are available in three sizes corresponding to a patient's height and clavicle length.
Additional plate options are also offered to address alternative clinical settings. The VA-LCP Clavicle Plate 2.7 System is the first system to include a dedicated medial plate designed to treat medial clavicular fractures (Fig 4). The XL shaft plate is designed for extended shaft fractures in larger patients' anatomies (Fig 5).
Each plate in the system is designed to reduce procedural complexity. The plate shapes match the bow and contour of the clavicle for low prominence and enhanced plate-to-bone fit. By improving overall plate fit, intraoperative plate placement is less challenging and the need for hardware removal due to irritation caused by prominent plates is reduced.
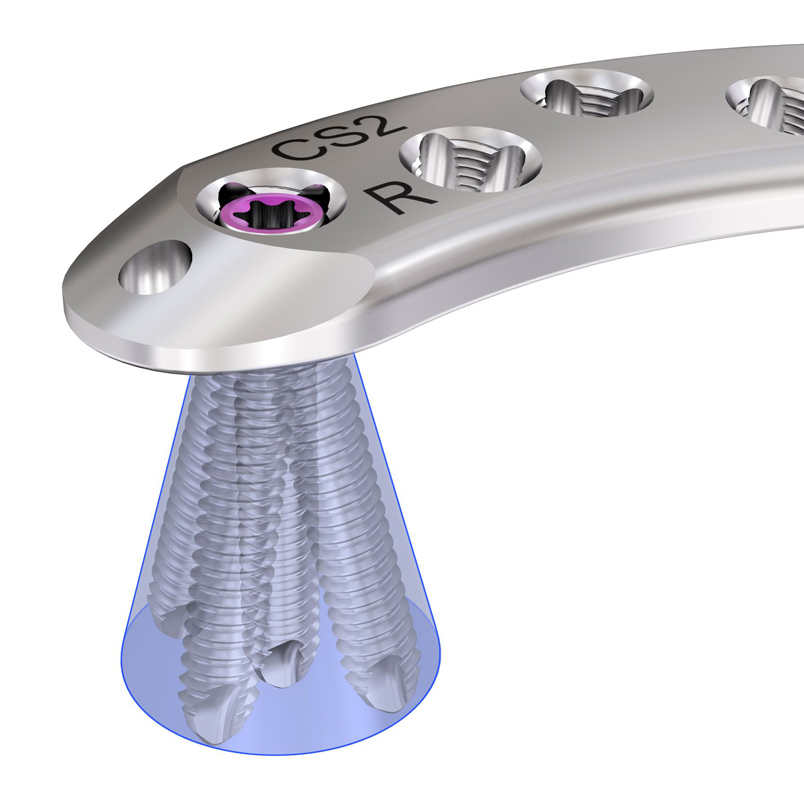
All screw holes in the VA-LCP Clavicle Plates accept 2.7 mm screws which presents an important adaptation to the previous LCP 2.7/3.5 mm Clavicle Systems. The decision to utilize only one screw size enhances usability through use of a single drill diameter for all screws. Metaphyseal screws with a low-profile head have also been included in the system to provide compression when needed. Staggered screw hole positioning with pre-defined hole angulation increases screw density (compared with a plate of the same length with in-line screw holes) and is designed to achieve the required construct stability (see white paper on the Mechanical Performance of the DePuy Synthes 2.7 mm VA-LCP Clavicle Plate System).
The VA Combi holes combine a dynamic compression unit with a VA locking hole. The VA Combi hole allows fixation with VA locking screws in the threaded section for angular stability and cortex screws in the non-threaded section for compression. The thread profile of the VA locking hole also allows for the angulation of the VA locking screw when needed by specific fracture patterns for fragment capture (Fig 6).
Smooth plate surface, tapered edges, and low-profile design with reduced thickness (compared with other clavicle systems) serve to further enhance the intraoperative experience and are features of the DePuy Synthes plate systems that are valued by surgeons during plate insertion.
VA-LCP Clavicle Hook Plate 2.7 System
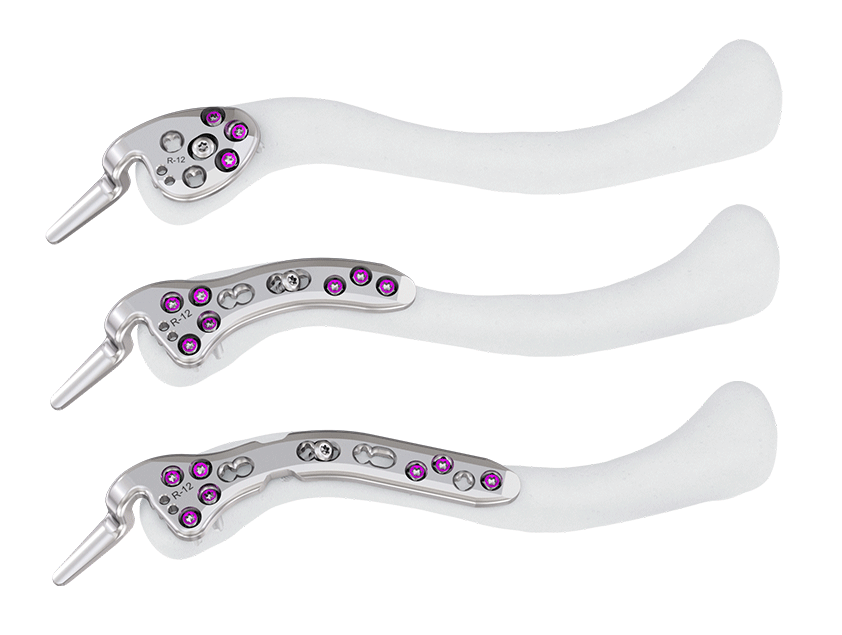
The system includes three low profile plate types (Fig 7); Button, Short and Long, with better anatomical fit and lower construct prominence intended to reduce soft- tissue irritation and related pain. Each plate type is available in left and right with three hook depths (9 mm, 12 mm, and 15 mm) to fit the angulation of the subacromial space in a wide variety of patients. The hook depth and angulation has been enhanced on all three plate designs following analysis of more than 120 shoulder CT scans.
The dedicated plate shapes not only possess an enhanced fit but also are designed to accommodate the bow and curvature of the clavicle at corresponding fracture locations. To achieve an optimal fit, a database of more than 600 CT clavicle scans was used to influence the design of the plate shapes while taking clavicle size as well as gender and ethnicity into consideration. For outlying anatomical variation, plates are designed to facilitate 3D bending.
The VA-LCP Clavicle Button Hook Plate 2.7 is the smallest hook plate on the market and has been specifically designed for the treatment of acromioclavicular joint separations. The shortened and rounded plate body allows the hook to be targeted at the optimal contact location under the acromion with minimal incision size (Fig 8). The 2.7 mm single screw (as seen in the VA-LCP Clavicle Plate 2.7 System) reduces system complexity and facilitates ease of surgical procedure.
The VA-LCP Clavicle Hook Plate 2.7 System has undergone several mechanical tests throughout development to assess plate construct and the performance of isolated design features, such suture holes. The construct pull-out strength is non-inferior in comparison with the DPS 3.5 mm LCP Clavicle Hook Plate System. Load testing was also completed to evaluate the performance of the system during plate contouring and hook bending.
Cases using VA-LCP Clavicle Plate 2.7 System
(by Martin Jaeger, Universitätsklinikum Freiburg, Germany)
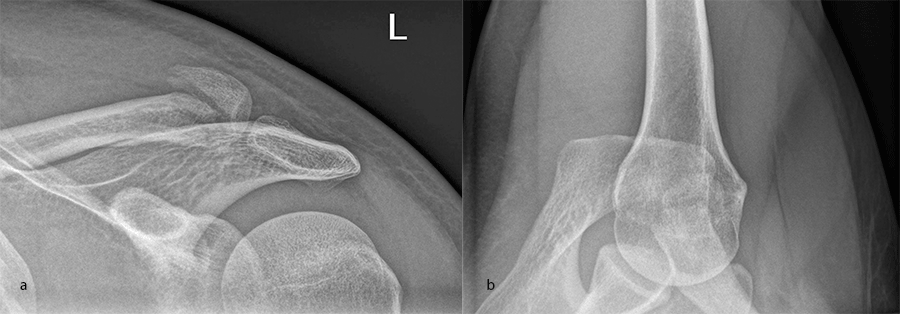
Case 1: lateral plate
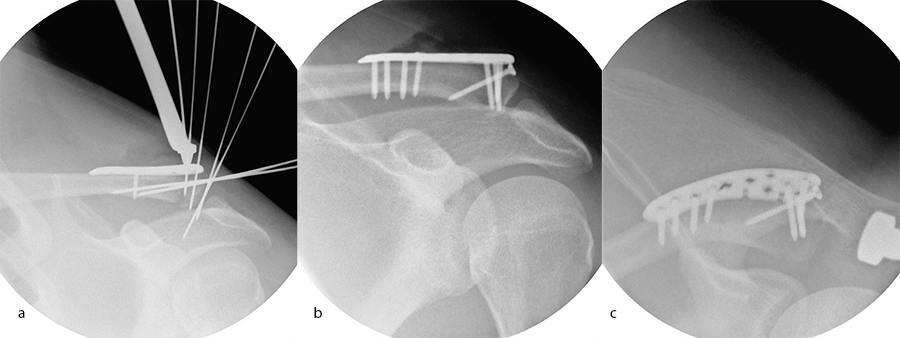
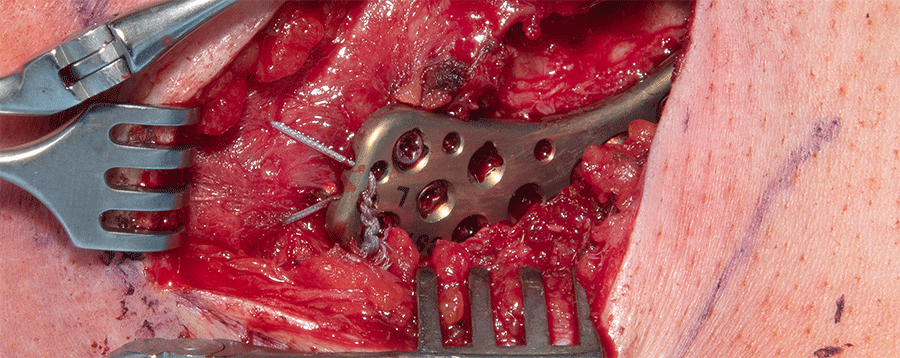
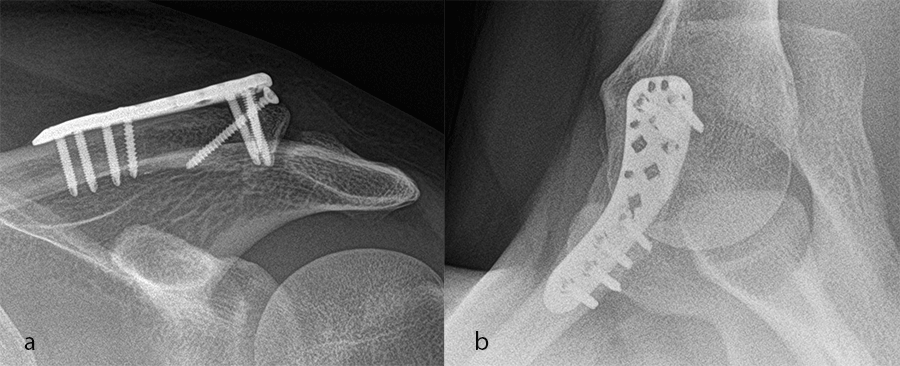
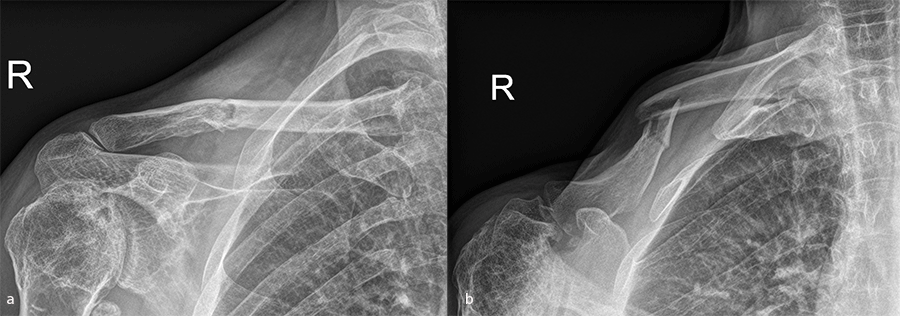
A 30-year-old man sustained a lateral fracture to his left clavicle following a fall from his bike (Fig 9). Intraoperative images indicate plate placement and screw insertion (Fig 10). Intraoperative image revealing usage of sutures through the plate for soft- tissue fixation (Fig 11). Image shows the fracture healing at 8 weeks' follow-up (Fig 12).
Case 2: shaft plate
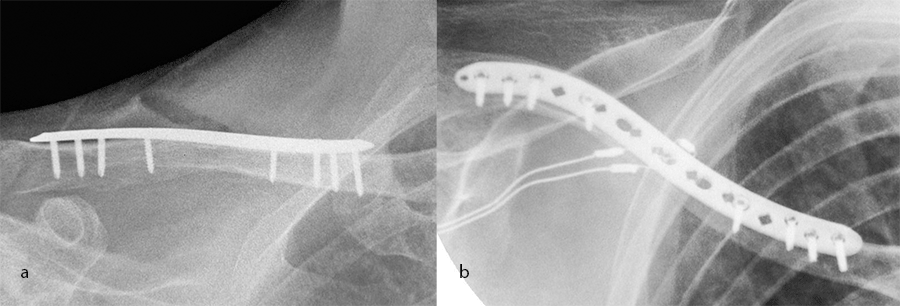
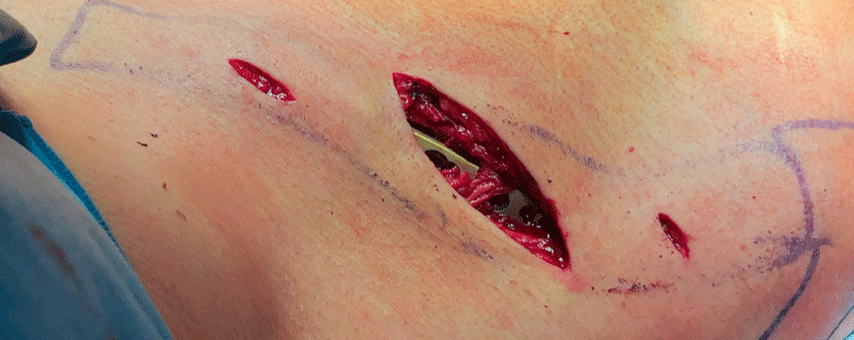
A 54-year-old man sustained a mid-shaft fracture to his right clavicle following a 2 m fall from a ladder (Fig 13). Intraoperative images show plate placement and screw insertion (Fig 14). Postoperative image depicting minimal incision size for plate insertion before wound closure (Fig 15). Image shows the fracture healing at 6 weeks’ follow-up (Fig 16).
Case 3: medial plate
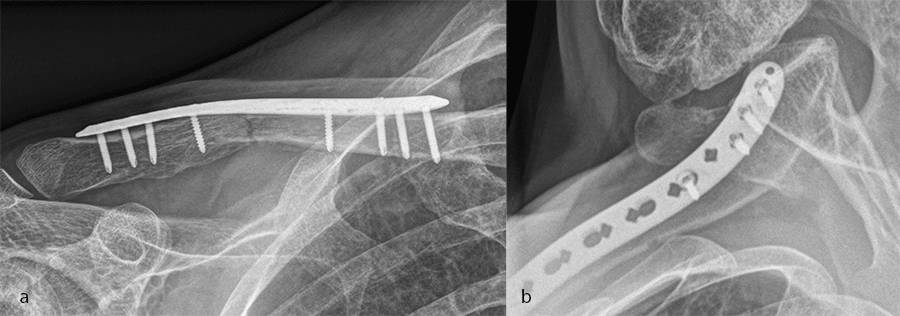
A 60-year-old woman sustained a medial fracture to the left clavicle following a car crash. A computed tomographic (CT) scan was performed 8 weeks after the incident (Fig 17). Intraoperative images reveal plate placement and screw insertion (Fig 18). Image shows the fracture healing at 4 weeks’ follow-up (Fig 19).
Innovations in clavicle fracture management: VA Clavicle System 2.7 mm - Segmental and hook plates
Presentation delivered by S. Lambert (UK) and M. Jaeger (DE), introducing the VA-LCP Clavicle 2.7 System.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.