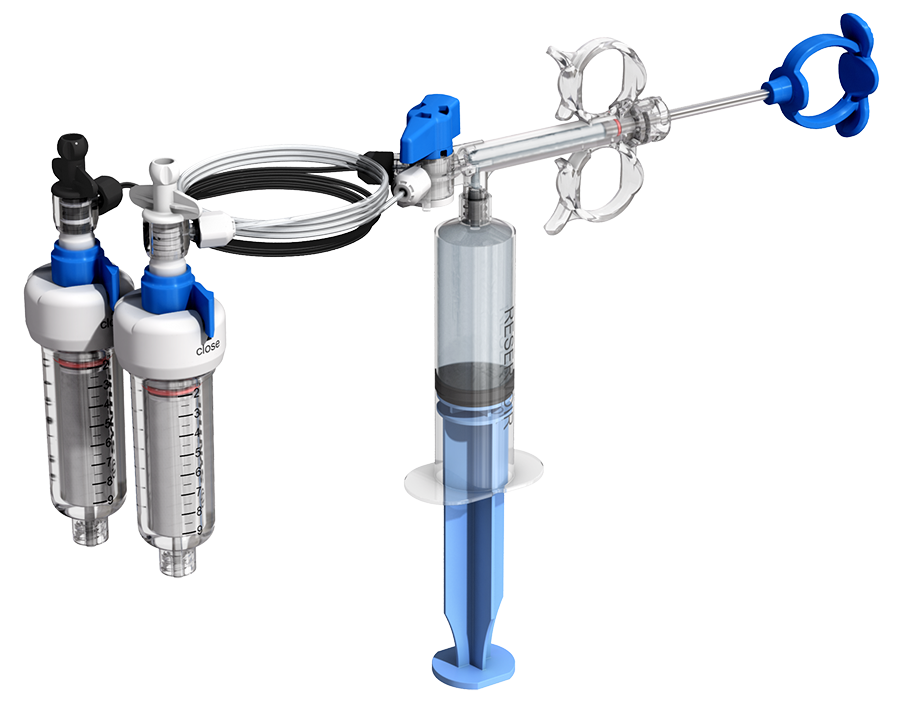
SYNJECT Cement Delivery System
The SYNJECT Cement Delivery System (CDS) is a hydraulic-based delivery system for the application of VERTECEM V+ bone cement to augment cancellous bone during vertebral body augmentation (kyphoplasty and vertebroplasty) and received its AO TC approval in 2021.
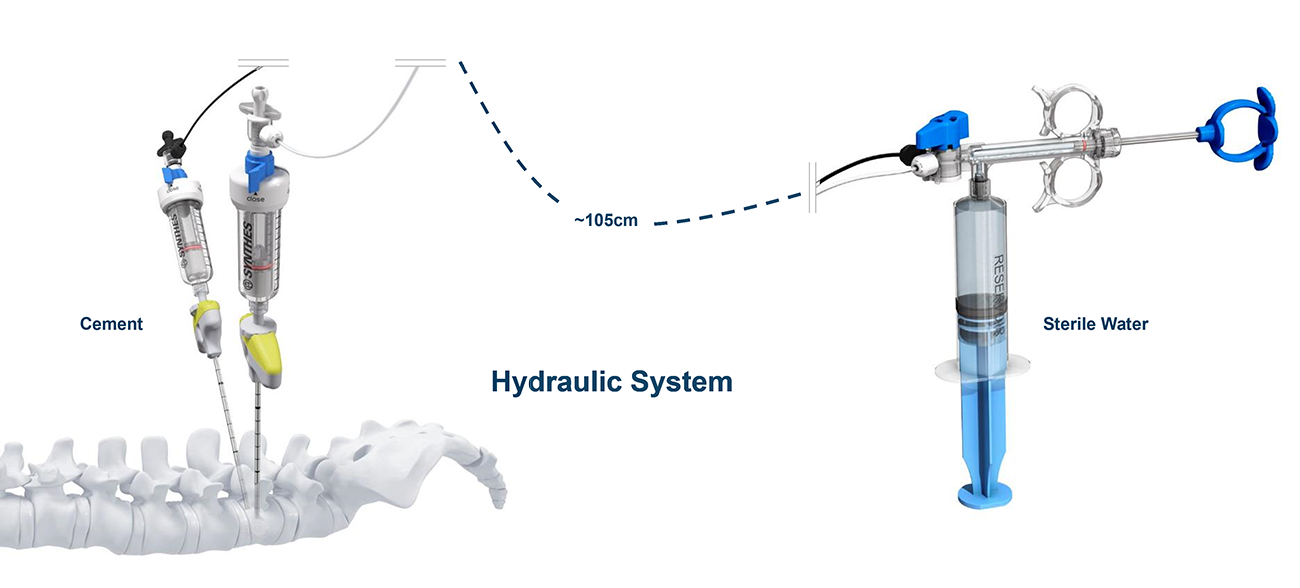
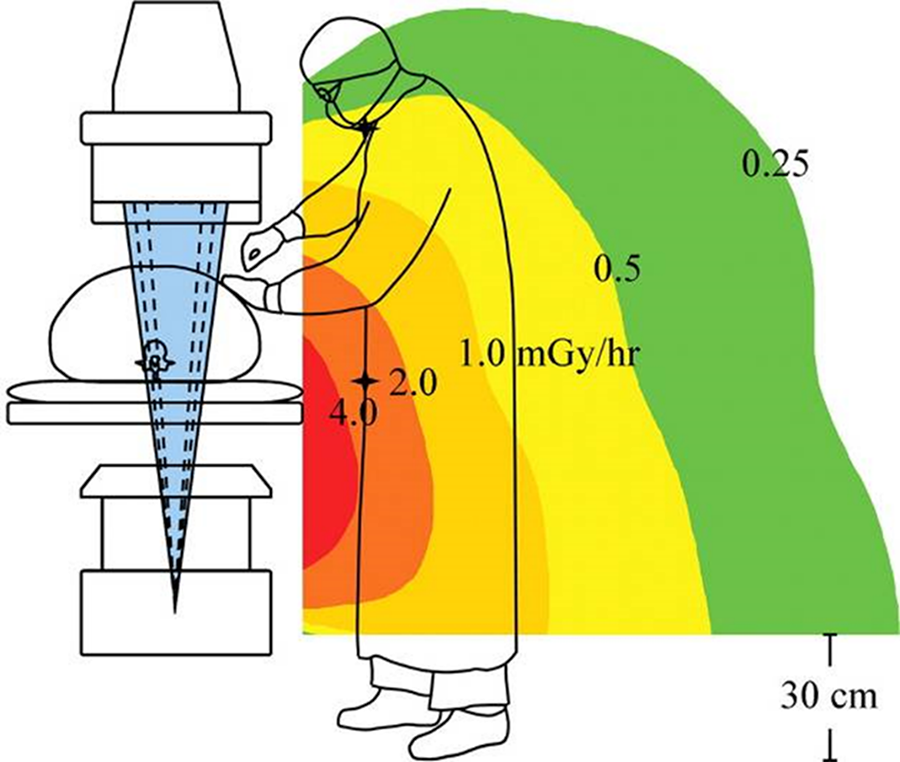
The design of SYNJECT is directly related to the clinical need to reduce radiation exposure to the surgeon during the cement injection and is allowing remote cement delivery.
Research has proven that spine surgeries, particularly kyphoplasty and vertebroplasty, expose operators to the highest radiation doses [1]. Too much radiation can lead to radiation-related illnesses, such as cataracts, thyroid disease, brain tumors, and brain disease [2–4].
To address the impact of high radiation on the health of spine physicians, SYNJECT has been designed with a remote delivery system to reduce radiation exposure from back scattered x-rays [Fig 2 and Fig 3].
The SYNJECT CDS provides sufficient pressure for the application of high-viscosity PMMA bone cement (VERTECEM V+) in a controlled way due to the hydraulic-based delivery and allows for the immediate stop of cement flow when required. This is effectively attained by releasing pressure on the syringe plunger (tactile feedback).
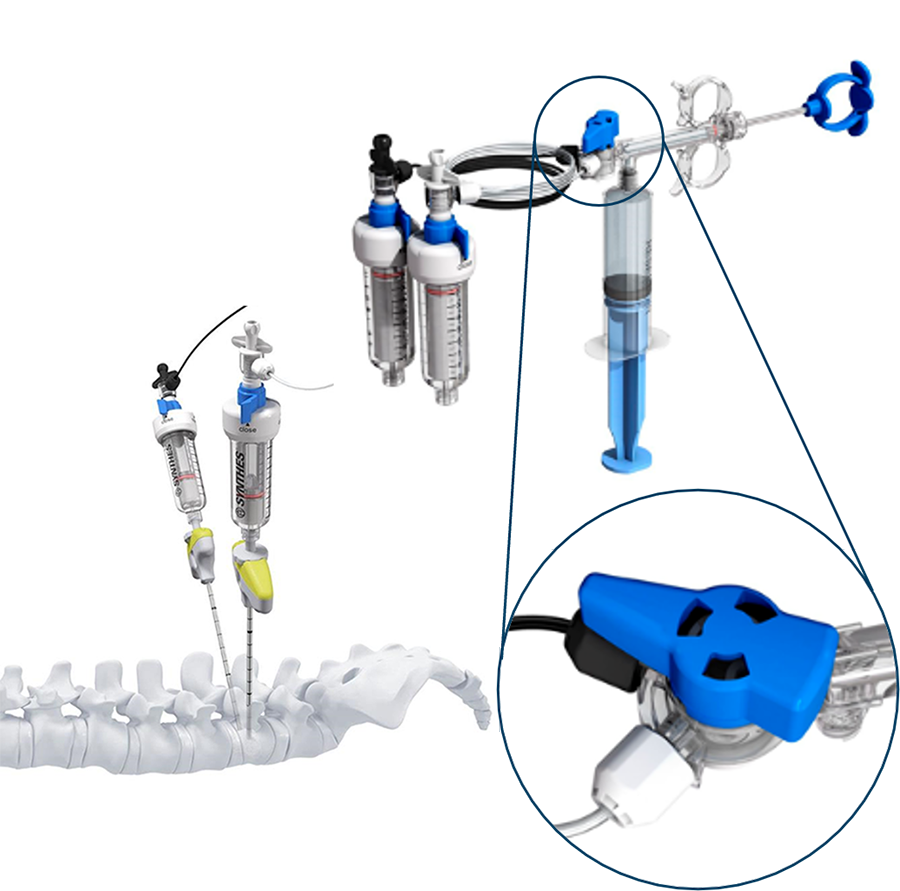
The system has an optional dual line injection system which is ideal for bi-pedicular cement application. During such procedures, which demand a bigger volume of cement, the ability to rapidly alternate between two injection needles in one device has a significant benefit in terms of time. The tubes and connectors in the SYNJECT dual line injection system are color coded which promotes ease of use and reduces the potential for confusion concerning the cement cartridges. Cement flow ceases during the switch between injection systems which assists with precise application [Fig 4].
During the design validation stage of the SYNJECT CDS, 100% of physicians rated both the pressure and tactile feedback of the system as very good.
References
- Efstathopoulos EP, Pantos I, Andreou M, et al. Occupational radiation doses to the extremities and the eyes in interventional radiology and cardiology procedures. Br J Radiol. 2011 Jan;84(997):70-77.
- Jacob S, Michel M, Spaulding C, et al. Occupational cataracts and lens opacities in interventional cardiology (O'CLOC study): are X-Rays involved? BMC Public Health. 2010 Sep;10:537.
- Jereczek-Fossa BA, Alterio D, Jassem J, et al. Radiotherapy-induced thyroid disorders. Cancer Treat Rev. 2004 Jun;30:369-84.
- Roguin A, Goldstein J, Bar O, et al. Brain and Neck Tumors Among Physicians Performing Interventional Procedures. Am J Cardiol. 2013 May;111(9):1368-1372.
- Lin E, Schueler B. Radiologic issues and radiation safety during ERCP. In: Ercp. 2019:14-29. Amsterdam: Elsevier.
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.