RIA 2 System: next generation reamer-irrigator-aspirator
Hans-Christoph Pape
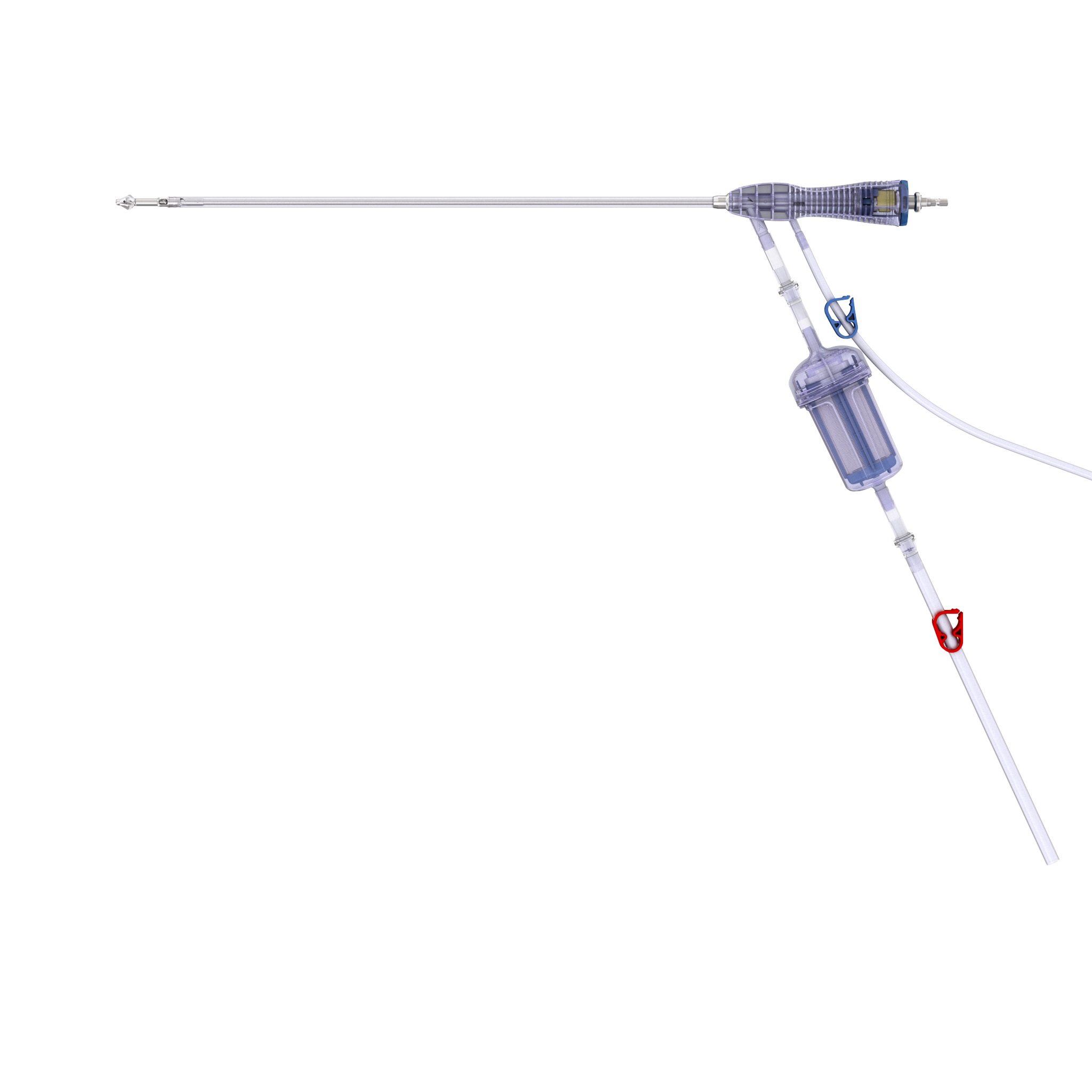
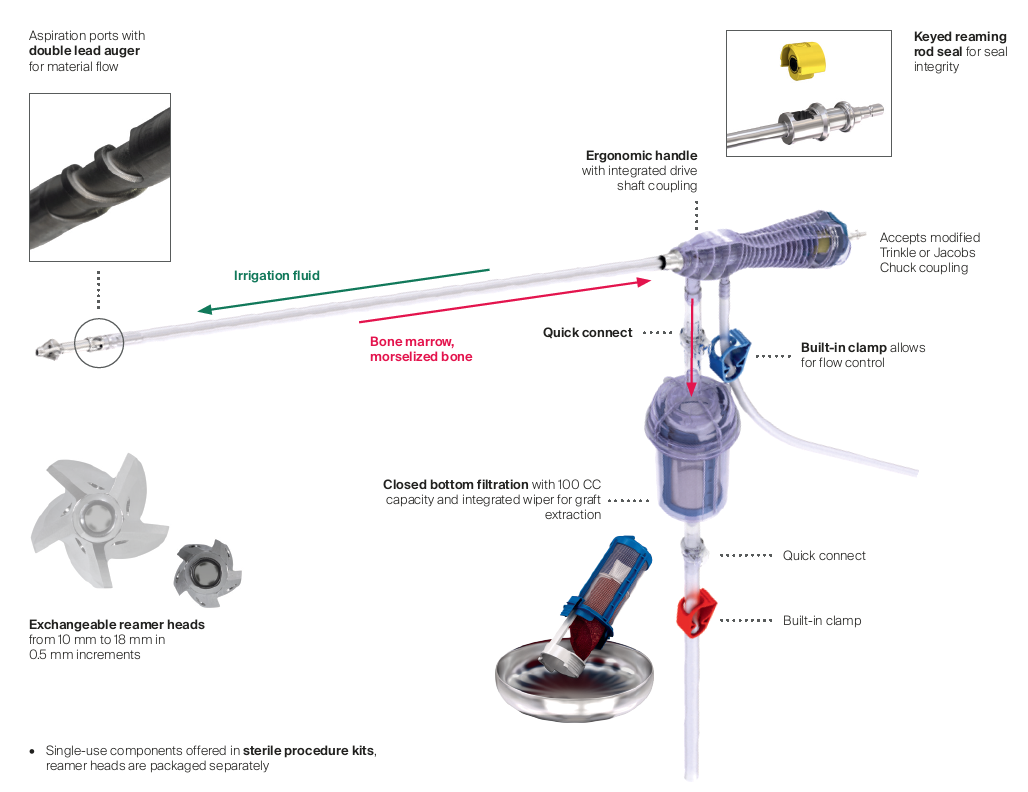
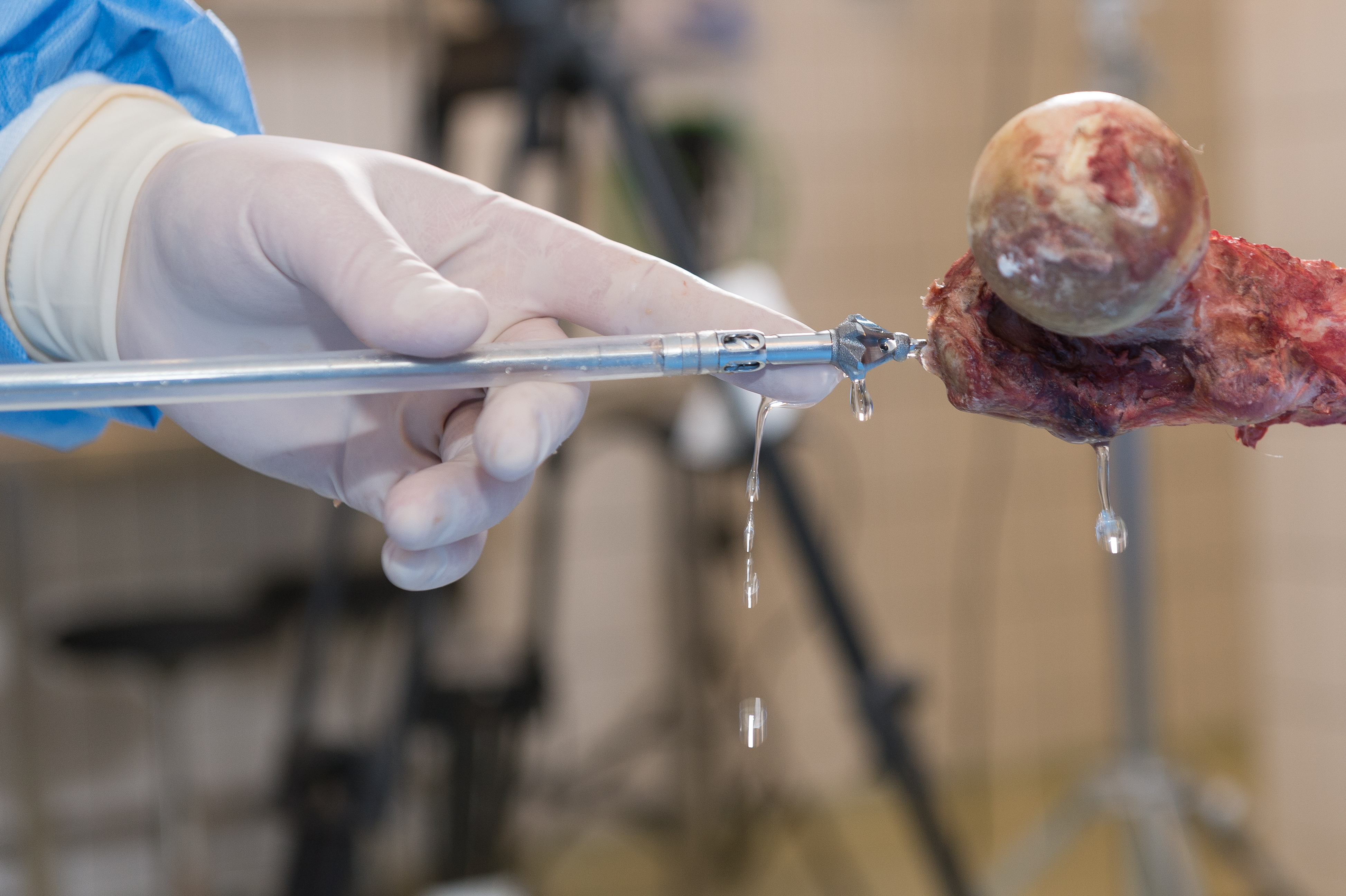
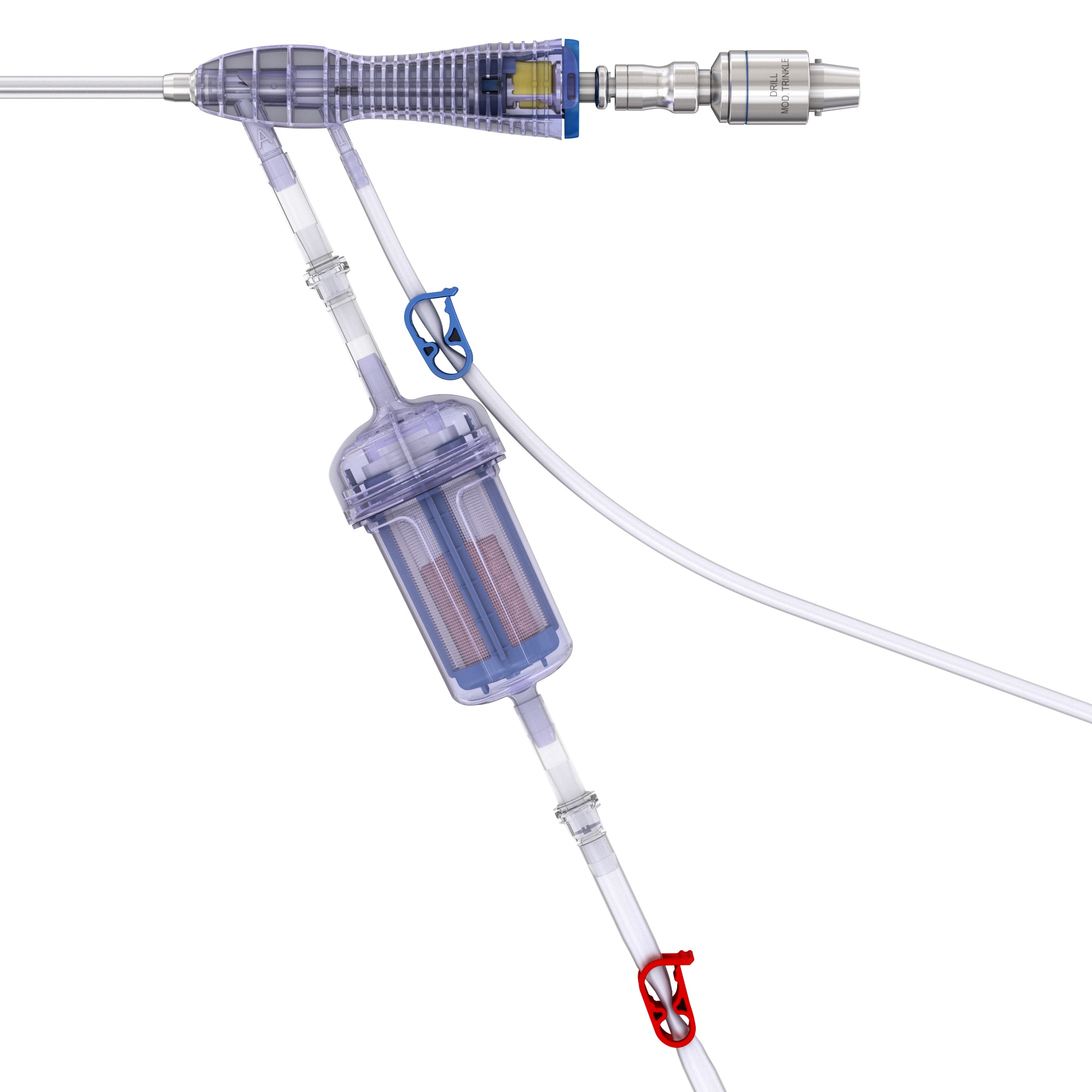
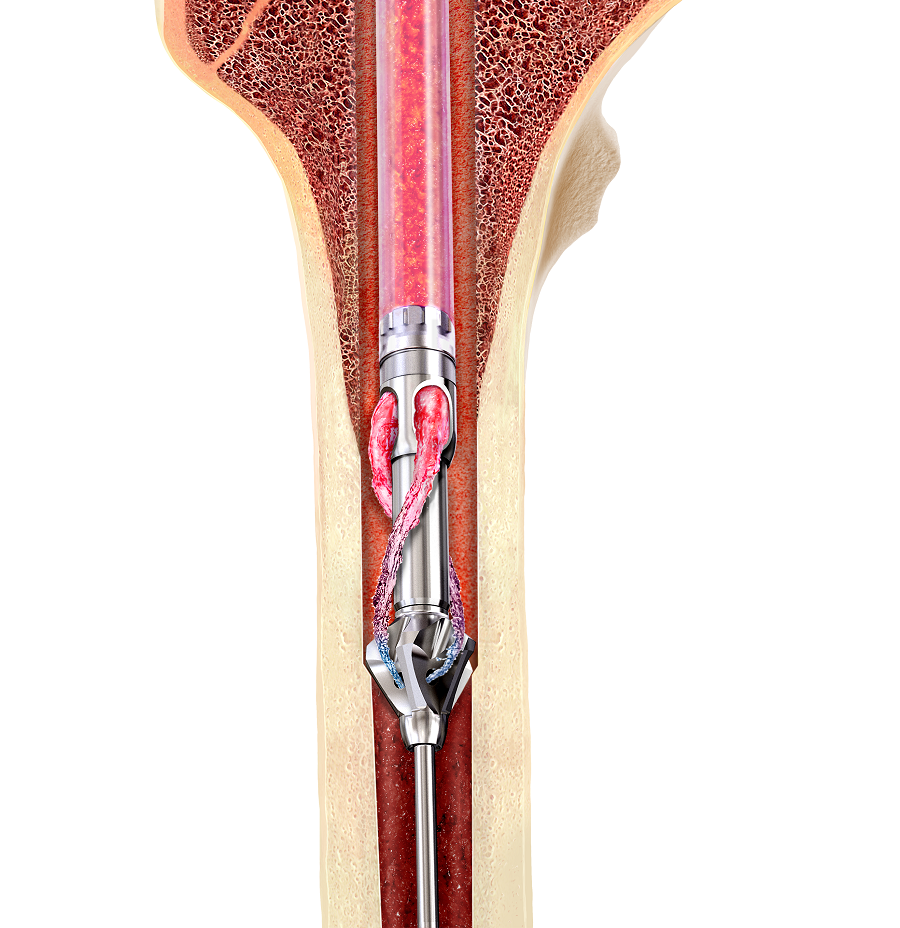
RIA 2 (Fig 1) is the next generation reamer-irrigator-aspirator device. Intended for use in adults and adolescents, its indications are to clear the medullary canal of bone marrow and debris, enlarge the medullary canal for the insertion of an intramedullary implant or prosthesis, to harvest morselized autogenous bone and bone marrow for bone grafting purposes, and to remove infected and necrotic bone and tissue from the medullary canal in the treatment of osteomyelitis. The RIA 2 system consists of a powered reamer with exchangeable cutter heads ranging from 10 mm to 18 mm in 0.5 mm increments (Fig 2). The reamer has integrated tubes for delivery of irrigation saline, and aspiration of tissue from the intramedullary cavity. The aspiration tube can be connected to a closed bottom filter, allowing the collection of bone graft material (Fig 2). The reamer heads, tube assemblies, drive shaft seals, and graft filters are designed for single-patient use only.
Note regarding reamer insertion: The guide wire entry angle must be less than 10° from the axis of the medullary canal. Otherwise, there is a risk that bowing of the reaming rod will result in eccentric reaming of the far cortex and damage to the reamer head connection, resulting in metal fragments in the canal. For more detailed information please refer to the surgical technique guide under Supporting Documents.
Improved features of RIA 2 compared to RIA 1
- Exchangeable reamer heads allow for intraoperative flexibility
- Smaller reamer head sizes allow improved access to previously limited anatomies, such as the tibia
- Sterile packed procedure kits improve efficiency by decreasing hospital inventory and preoperative complexity
- Designed for simplified assembly and handling
Advantages of RIA 2
RIA 2 offers many advantages over conventional reaming and addresses several unmet clinical needs, as outlined below.
Efficient harvest of autograft
Bone grafting is a common adjunct in the management of many traumatic and reconstructive orthopedic procedures [1]. Approximately 500,000 bone graft harvesting procedures are undertaken every year in the United States [1].
Autogenous bone is considered to be the gold standard bone grafting material [2]. It is osteoinductive (containing bone morphogenetic proteins [BMPs] and other growth factors), osteogenic (due to the presence of osteoprogenitor cells) and osteoconductive (providing a scaffold) [3]. Autogenous bone is widely used for augmentation and acceleration of bone regeneration, and for the restoration of bony defects [2].
To date, the most common harvest site for autogenous bone graft is the iliac crest [2], but this necessitates an additional surgical procedure with well-documented complications and discomfort for the patient. Complication rates of up to 30% have been reported from iliac crest bone grafts [4]. Potential complications include infection, hematoma/seroma, fracture, nerve and vascular injuries, chronic donor site pain, hernias, unsightly scars, and poor cosmetic outcome [2]. These complications are associated with substantial costs, including prolonged hospitalization.
The RIA System (Figs 2, 4 and 5) provides an efficient method for obtaining large volumes of autogenous bone graft. Reaming a medullary canal using the RIA device is clinically proven to have lower complication rates and reduced donor site pain compared to iliac crest bone graft (ICBG).
The RIA system harvests 3848 cc mean volume of graft per procedure [46]. In a comparative study conducted by Dawson et al, the RIA system produced 17 cc more graft on average than anterior ICBG [5]. Harvesting bone graft with RIA reduces the rate of complications from 19.4% to 6.0% [2] and significantly reduces donor site pain (P < .004) compared to ICBG [5]. Union rates and time to union are better [7,8] or comparable [5] comparing the RIA system to ICBG. The RIA system produces bone graft with concentration of viable cells and growth factors better or consistent with ICBG [9]. The use of RIA autograft allows hospitals to realize cost savings, as it is less expensive than both demineralized bone matrix (DBM) allograft and bone morphogenetic protein (per/10 cc) [5]. Furthermore, studies comparing bone healing in patients treated with autograft or allograft have shown a comparable or better time to union and union rates with autograft [10].
Fewer complications during reaming
Despite the widespread acceptance of standard reaming, there are well-documented complications and adverse events associated with its clinical use. The incidence of heterotopic ossification with the use of standard reaming has been reported to be as high as 35.7% [11]. Further complications include fat embolism syndrome, adult respiratory distress syndrome, sudden intraoperative deaths and aseptic cortical thermal necrosis [12]. Additionally, the use of standard reaming for the insertions of nails for impending and pathological fractures secondary to metastatic cancer may additionally cause systemic embolization of malignant cells, potentially increasing the rate of distant metastasis [13].
RIA (Fig 4) offers a solution to many of the complications associated with standard reaming. Reaming with RIA has been reported to reduce the risk of heterotopic ossification: incidences of heterotopic ossification for the RIA System have been reported to be between 0 and 0.86% [2, 14]. The RIA system significantly reduces the total emboli score during reaming (P < .05) and nail insertion (P < .05) compared to standard reaming [15], thereby decreasing pulmonary insult. Furthermore, RIA has been demonstrated to reduce heat generation compared to standard reaming and may therefore reduce the risk of thermal necrosis [16]. By reducing such complications, RIA offers cost savings for hospitals and better patient outcomes.
Management of infection
Medullary cavity infection can delay healing of long bones and often requires surgery and prolonged medical treatment, resulting in increased morbidity for the patient, and often a poor functional result. While standard reaming of the medullary canal for the debridement of the infected cavity has been shown to be a beneficial method of infection control [17], it can be associated with an increased risk of thermal necrosis, a lack of control of infected bone particles, and the risk of infected material propagation [18]. The RIA system is an effective adjunct in the treatment of long-bone osteomyelitis [17]. A study conducted by Zalavras et al [19] demonstrated no recurrence of osteomyelitis of the tibia and femur when the RIA System was used for intramedullary canal debridement.
Reduced procedure time
Use of RIA may provide economic benefits to hospitals by way of reduced length of hospital stay and operating time. Patients with bone graft harvested via the RIA System (Figs 5a-b) have a shorter duration of operation (P < .0001) and length of stay (P < .0001) compared to patients who had autogenous anterior ICBG [7]. Additionally, the RIA System significantly increases the chances that patients leave on the day of surgery versus patients treated with ICBG [20].
Improved patient outcomes
Although most fractures heal uneventfully, nonunions remain a relatively common problem [5]. Although ICBG has long been the gold standard source of autograft used in the treatment of nonunions, several studies have reported better or comparable union rates and time to union with the RIA System vs ICBG [58]. Bone graft harvesting can result in complications, such as residual pain and nerve injury. Chronic pain is the most common complication with ICBG [2]. Studies comparing pain after bone graft harvesting have shown that patients treated with RIA had significantly lower pain scores than those patients treated with ICBG [5, 21], and a lower incidence of chronic pain at follow-up [8].
Summary
RIA 2 provides an efficient method for clearing the intramedullary cavity of debris and obtaining large volumes of autogenous bone graft. It reduces complication rates compared to ICBG harvesting, decreases pulmonary insult and thermal necrosis in comparison to standard reaming, and offers cost- saving opportunities for hospitals.
The development process for RIA 2
Convened in 2013, the RIA Task Force (RIATF) is a dynamic group of nine internationally renowned trauma and orthopedic surgeons, tasked with providing expert clinical guidance throughout the development of RIA 2.
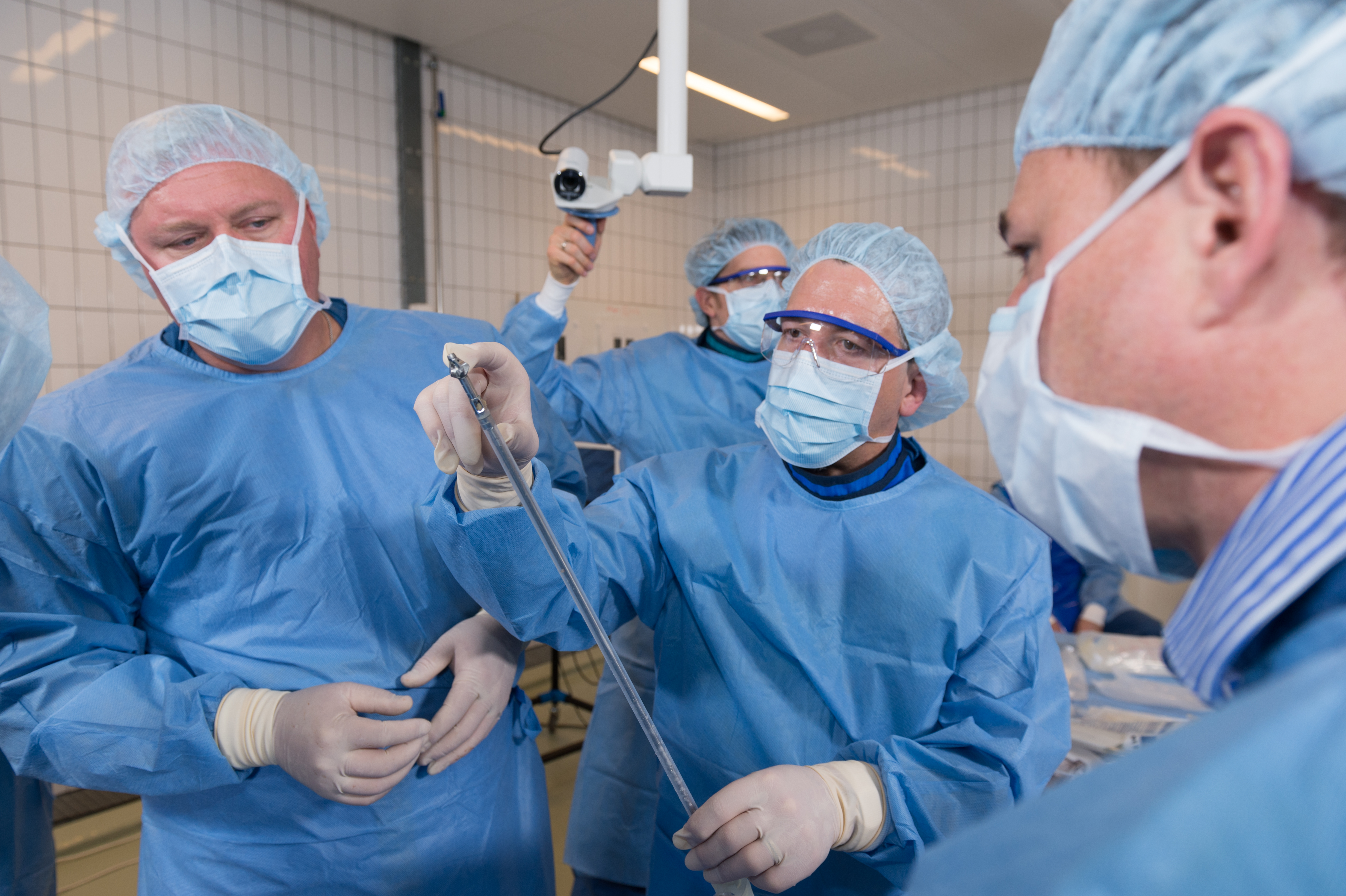
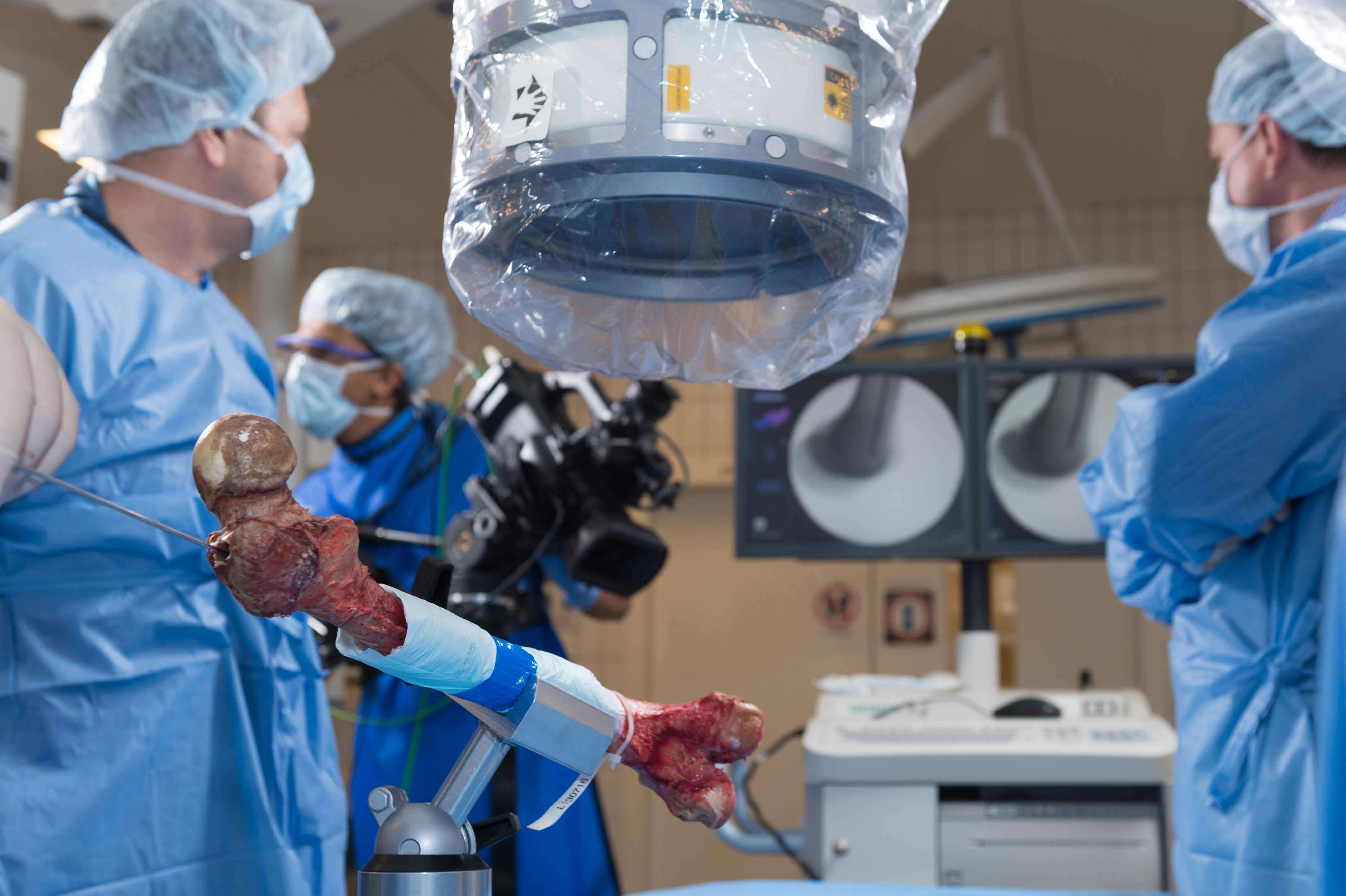
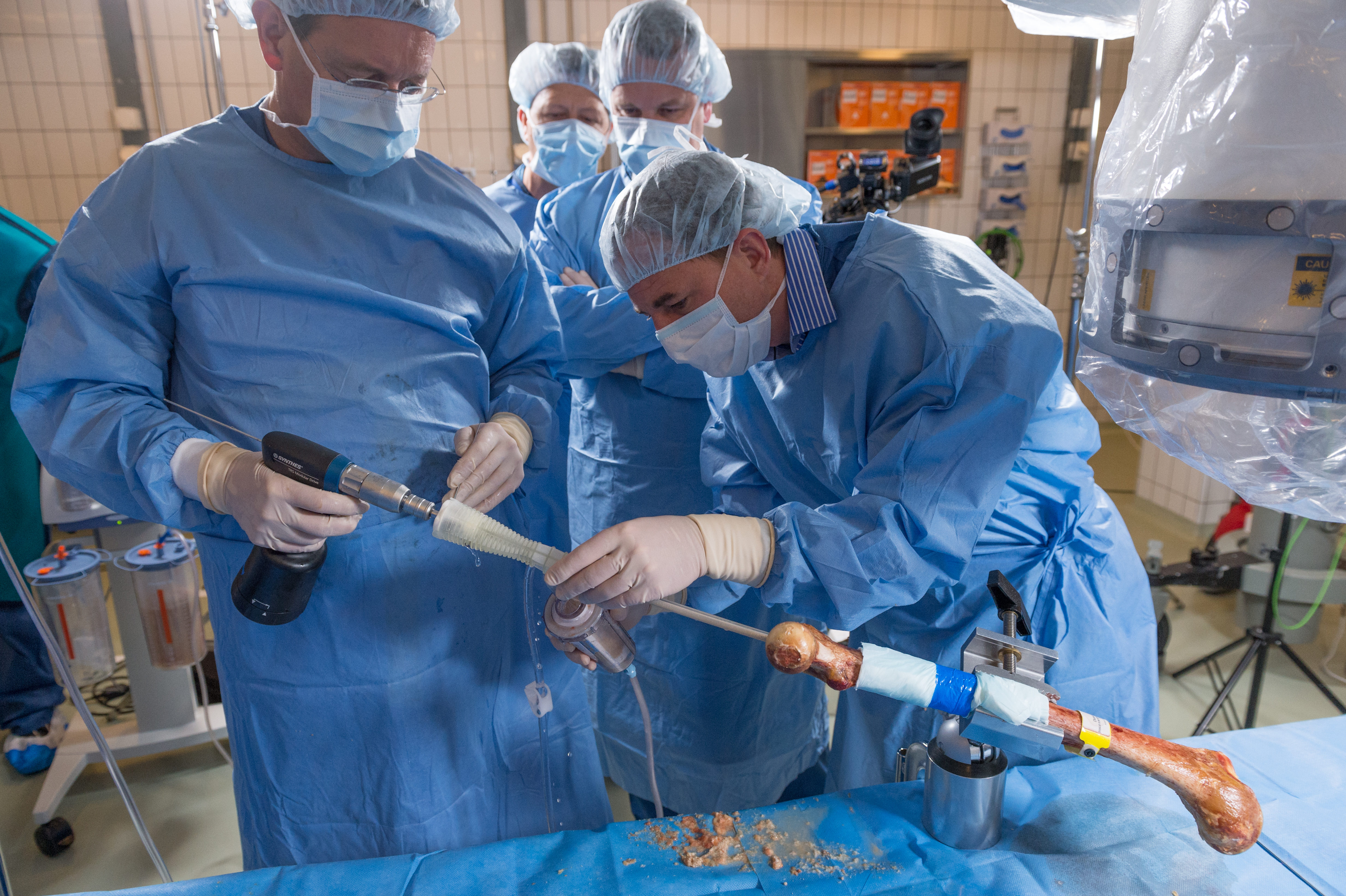
The Task Force has been active in testing the next generation RIA prototypes and guiding technical improvements from a global clinical standpoint. Medical members of the RIATF have undertaken testing of the device in anatomy laboratories in Davos (December 2015) and Solothurn (May 2016), Switzerland; West Chester, Pennsylvania (October 2016, April 2017, and April 2018), and in Orlando, Florida (October 2018), USA. These usability laboratories are a critical part of the development process (Fig 3a,b and Fig 6a,b).
The RIATF is at the forefront of evidence creation relating to indications for RIA usage, complications associated with RIA usage, and biomechanical considerations during reaming. Past and present medical members of the RIATF are heading up several research studies focusing on the RIA System:
- Retrospective clinical study regarding RIA indications and complications (Christoph Mller)
- Biomechanical investigation of the influence of the reaming diameter (RIA) on failure loads of human femora [22] (Michael Raschke)
- Biological properties of harvest material [9] (Ingo Marzi)
- Animal polytrauma study to establish local and systemic safety of RIA 2 (Hans-Christoph Pape).
Additionally, the RIA Task Force seeks to educate orthopedic surgeons throughout the world in conjunction with AOTrauma, to facilitate successful use of the new device. The RIATF members have produced educational material (Frequently Asked Questions about the Reamer Irrigator Aspirator system, Fig 7, and Annotated Reamer Irrigator Aspirator Abstracts), which will feature in the curricula of AO Courses on IM nailing, infection, and orthogeriatrics.
A webinar entitled "Using the RIA without complications" was presented by Prof Peter Giannoudis in Switzerland on September 2016. A webcast highlighting the new features of RIA 2 will be offered in 2020. A video demonstrating complication-free use of the RIA device both in bone models and in the operating room will be available later in 2019.
The RIA Task Force Chair, Chris Pape, says:
"Advances in the surgical treatment of trauma have generated the need for more sophisticated RIA instrumentation. The new generation RIA device will allow surgeons to continue to improve outcomes in complex cases where patients require reaming and bone grafting".
What next for the RIATF?
Medical members of the RIATF (Fig 8) recently received funding approval for a new international, multicenter, prospective registry to investigate treatment options and their outcomes on long-bone defects. The study aims to recruit 600 cases during a 3-year enrollment period starting in the fourth quarter of 2019. Exploratory analyses will be conducted to investigate relationships between the different types (categories) of bone defects, treatment methods, complications, and outcomes.
While the RIA Task Force will be disbanded at the end of 2019 once RIA 2 is launched, selected members will offer expert clinical guidance for a new project to develop and validate a long-bone graft cage. The graft cage is a biodegradable device to hold bone graft tissue in situ for the duration of healing of long-bone defects. Animal studies show better healing in long-bone defects treated with graft cage versus existing methods, and the aim is to extend this benefit to the clinical setting.
References
1. Greenwald AS, Boden SD, Goldberg VM, et al. Bone-graft substitutes: facts, fictions and applications. J Bone Joint Surg Am. 2001;83-A Suppl 2 Pt 2:98103.
2. Dimitriou R, Mataliotakis GI, Angoules AG, et al. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury. 2011;42-Suppl 2:S3S15.
3. Bauer TW, Muschler GF. Bone graft materials. An overview of the basic science. Clin Orthop Relat Res. 2000 Feb;(371):1027.
4. Herscovici D Jr, Scaduto JM. Use of the reamer-irrigator-aspirator technique to obtain autograft for ankle and hindfoot arthrodesis. J Bone Joint Surg Br. 2012 Jan;94(1):7579.
5. Dawson J, Kiner D, Gardner W 2nd, et al. The reamer-irrigator-aspirator as a device for harvesting bone graft compared with iliac crest bone graft: union rates and complications. J Orthop Trauma. 2014 Oct;28(10):584590.
6. Qvick LM, Ritter CA, Mutty CE, et al. Donor site morbidity with reamer-irrigator-aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013 Oct;44(10):12631269.
7. Flecher X VJ, Parratte S, Argenson JNA. Can reamer-irrigator-aspirator replace the iliac autografting in diaphyseal long bone nonunion? American academy of orthopedic surgeons 2014 annual meeting; March 11-15, 2014 New Orleans, LA, USA.
8. Nodzo SR, Kaplan NB, Hohman DW, et al. A radiographic and clinical comparison of reamer-irrigator-aspirator versus iliac crest bone graft in ankle arthrodesis. Int Orthop. 2014 Jun;38(6):11991203.
9. Henrich D, Seebach C, Sterlepper E, et al. RIA reamings and hip aspirate: a comparative evaluation of osteoprogenitor and endothelial progenitor cells. Injury. 2010 Nov;41 Suppl 2:S6268.
10. Flierl MA, Smith RW, Mauffrey C, et al. Outcomes and complication rates of different bone grafting modalities in long bone fracture nonunions: a retrospective cohort study in 182 patients. J Orthop Surg Res. 2013 Sep 9;8:33.
11. Furlong AJ, Giannoudis PV, Smith RM. Heterotopic ossification: a comparison between reamed and unreamed femoral nailing. Injury. 1997 Jan; 28(1):914.
12. Kanakaris NK, Morell D, Gudipati S, et al. Reaming irrigator aspirator system: early experience of its multipurpose use. Injury. 2011 Sep;42 Suppl 4:S28S34.
13. Cipriano CA, Arvanitis LD, Virkus WW. Use of the reamer-irrigator-aspirator may reduce tumor dissemination during intramedullary fixation of malignancies. Orthopedics. 2012 Jan 16;35(1):e4852.
14. Quintero A, Tarkin I, Pape H. Technical tricks when using the reamer irrigator aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24(1):4245.
15. Hall JA, Vicente MR, Morison ZA, et al. A prospective randomized trial investigating the effect of the reamer-irrigator-aspirator (RIA) on the volume of embolic load and respiratory functions during intramedullary nailing of femoral shaft fractures. Paper presented at: Orthopedic Trauma Association Annual Meeting; October 912, 2013; Phoenix, Ariz, USA.
16. Higgins TF, Casey V, Bachus K. Cortical heat generation using an irrigating/aspirating single-pass reaming vs. conventional stepwise reaming. J Orthop Trauma. 2007;21(3):192197.
17. Pape HC, Zwipp H, Regel G, et al. Chronic diaphyseal osteomyelitis of long bones refractory to conventional therapybenefits and risks of reaming of the femoral medullary cavity. Eur J Orthop Surg Traumatol. 1995 Dec;5(1):5358.
18. Tosounidis TH, Calori GM, Giannoudis PV. The use of the reamer-irrigator-aspirator in the management of long bone osteomyelitis: an update. Eur J Trauma Emerg Surg. 2016;42:417423.
19. Zalavras CG, Singh A, Patzakis MJ. Novel technique for medullary canal debridement in tibia and femur osteomyelitis. Clin Orthop Relat Res. 2007;461:3134.
20. Miladore M NS, Rohrbacher B, Ritter C. Early postoperative outcomes of different bone graft harvesting techniques for tibiotalar arthrodesis. Paper presented at: Orthopedic Trauma Association Annual Meeting; October 710, 2015; San Diego, Calif, USA.
21. Calori GM, Colombo M, Mazza EL, et al. Incidence of donor site morbidity following harvesting from the iliac crest or RIA graft. Injury. 2014;45 Suppl 6:S116120.
22. Schmidz N, Gehweiler D, Whnert, D, et al. Influence of the reaming diameter on failure loads of human femora. Paper presented at: Deutscher Kongress fr Orthopdie und Unfallchirurgie (DKOU); October 2326, 2018; Berlin.
Case 1: RIA 2 use for bone grafting at the tibia (provided by Brent Norris, Tulsa, US)
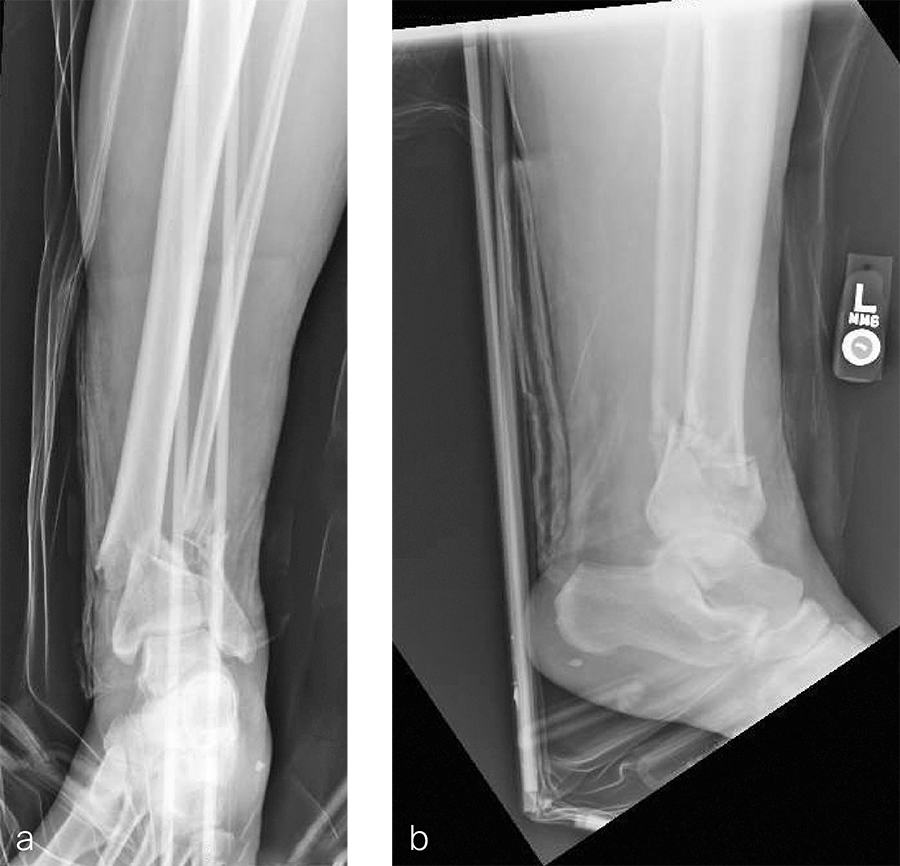
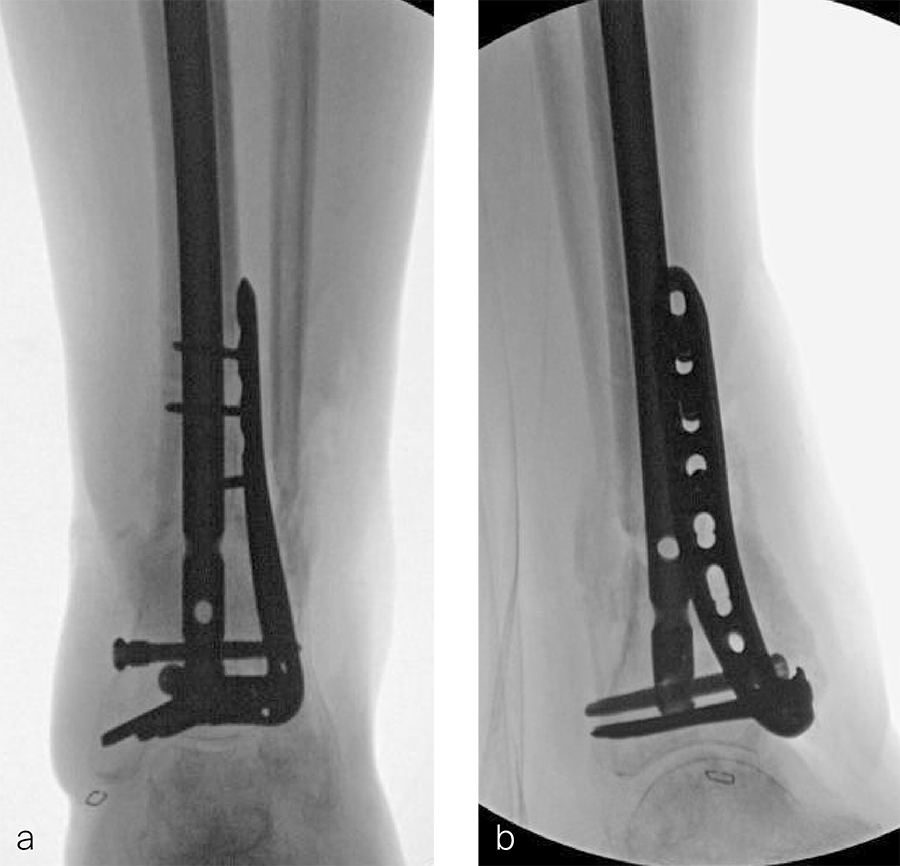
A 59-year-old man working for the city of Tulsa suffered an open distal tibia plafond fracture on the left leg when he fell into a sewer hole with raw sewage (Fig 1). The wound was grossly contaminated, and he was taken immediately to the operating room (OR) for incision and drainage and application of a spanning external fixator. Bone loss was noted anterior and medial (about 2.5 or 3 cm) but only about 2025% of the bone circumference.
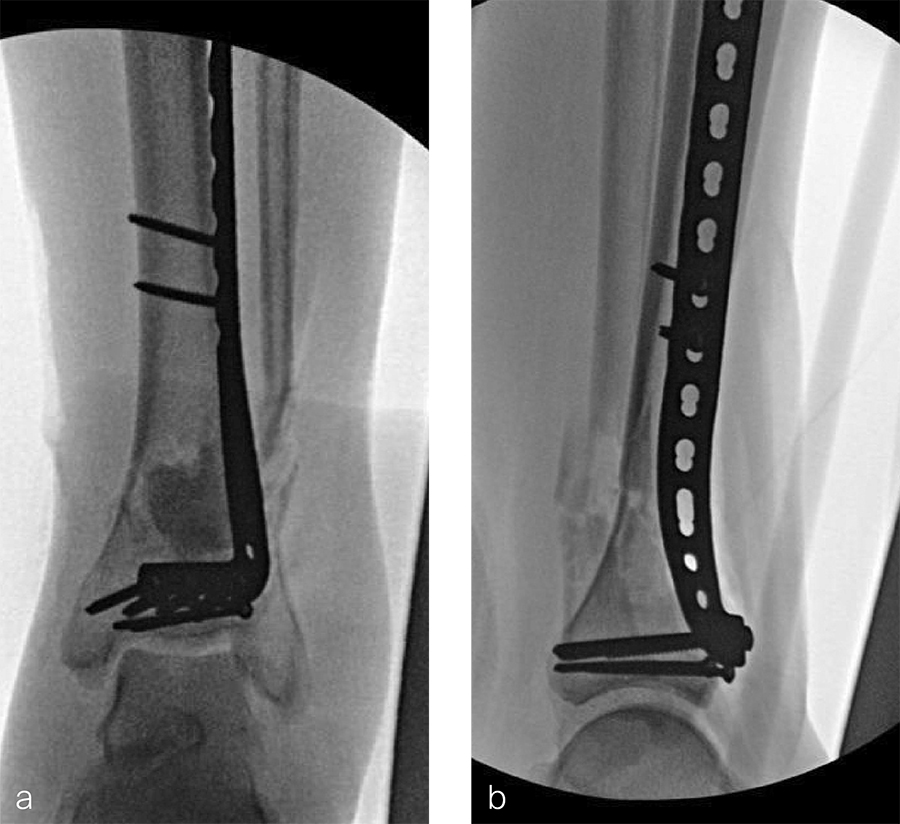
After repeated incision and drainage, 2 days later the fracture was repaired with an anterior lateral tibial plafond plate (Fig 2). Vancomycin and tobramycin impregnated antibiotic beads were placed in the bone defect.
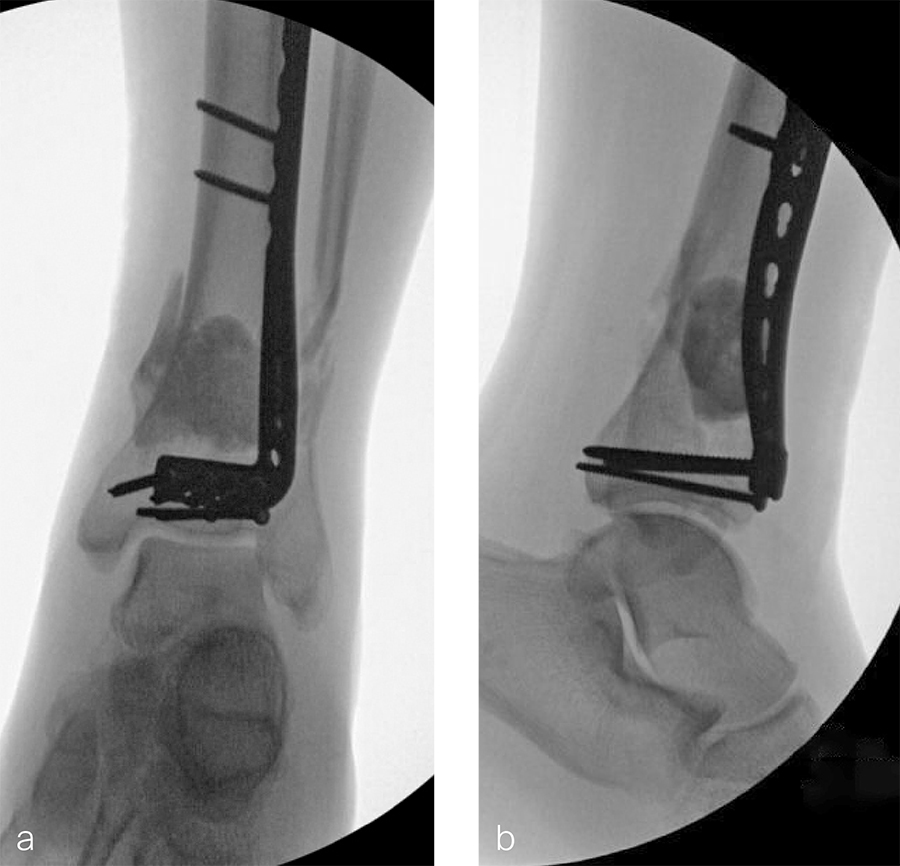
The patient was discharged on hospital day 6 to a rehabilitation facility with his left lower extremity in a splint and touch down weight bear allowance. He returned 2 weeks later with drainage from the medial traumatic wound. In addition, he had a gastrointestinal bleed from use of nonsteroidal antiinflammatory drugs. Once the gastrointestinal bleed was stabilized, he was taken the next day to the OR for another incision and drainage. Further, more significant, devitalized bone was resected, and an antibiotic cement spacer was placed (Fig 3). Deep culture samples were taken despite knowing the antibiotic resorbable beads had been placed in the wound at the time of initial closure. The plate was left in place, but a planned exchange plate/nailing was to be performed pending final culture results.
Cultures eventually yielded Klebsiella pneumoniae and Enterobacter cloacae. The patient was administered intravenous antibiotics for 6 weeks and was discharged home with therapy and nursing. He returned to the clinic with wound breakdown and an exposed cement spacer at 10 weeks after injury. Further bone debridement, spacer exchange, repeated culture samples, and plastic surgery were undertaken to help with wound coverage with a rotational flap. The microbiological culture was still positive for Klebsiella pneumoniae.
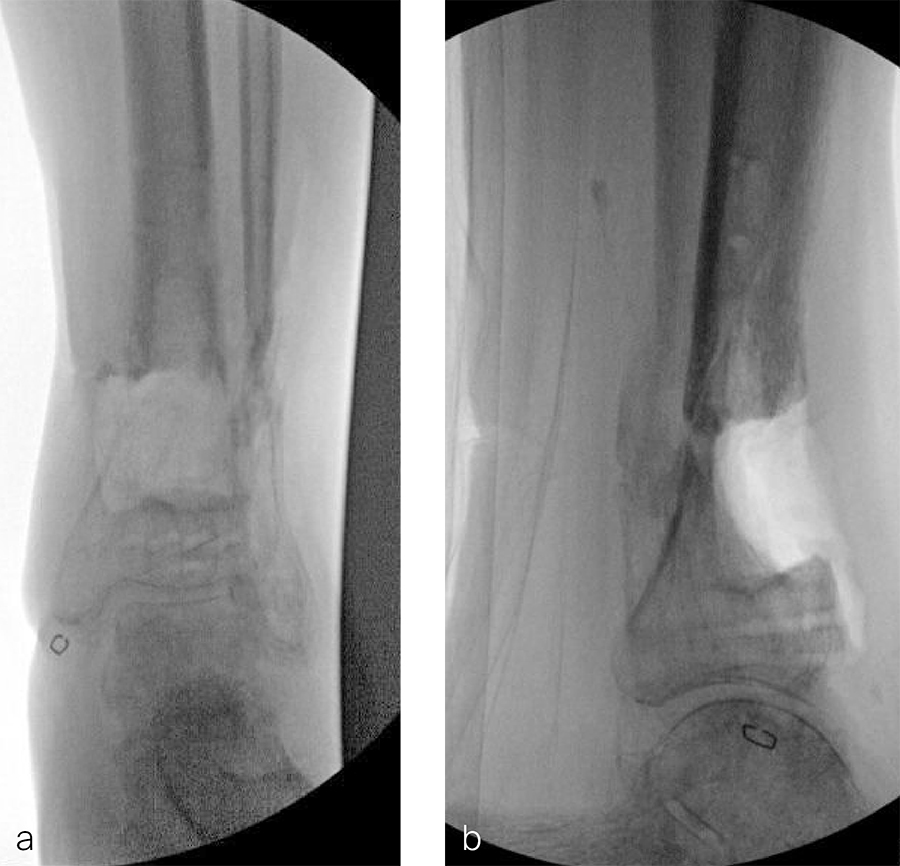
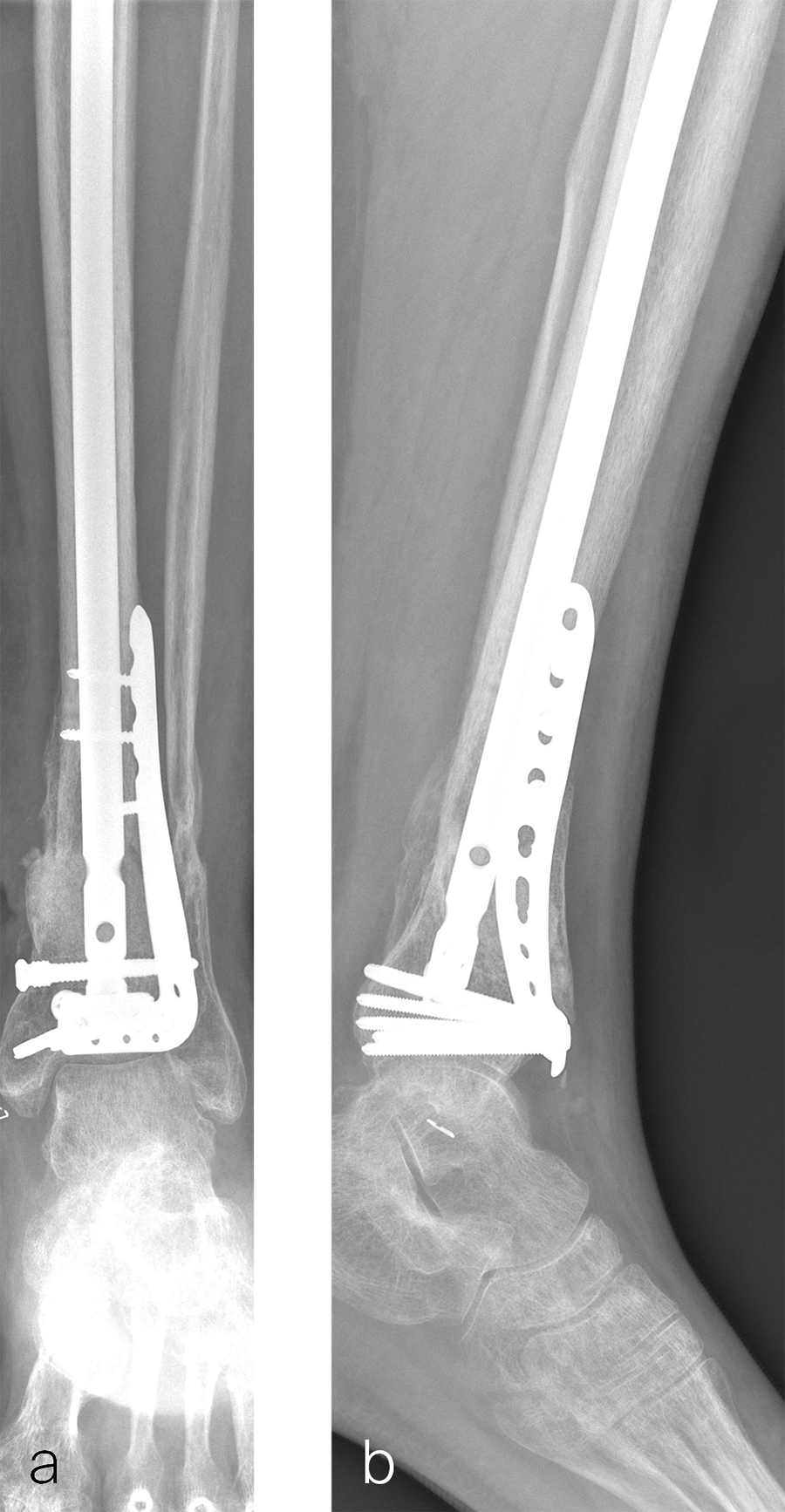
Two months later, the patient had a staged cement spacer removal and hardware removal followed by a new spacer placement (Fig 4). When microbiological cultures were negative for 5 days he was taken to the OR for definitive fixation and bone grafting. RIA bone graft from the ipsilateral femur was taken, a new anterior lateral plate was placed with an adjunct IM nail (and angle stable screws) as the distal plafond was now one articular block (Fig 5).
The patient has been followed up monthly for more than 5 months and has shown continued consolidation of the bone defect (Fig 6). Furthermore, he is weight bearing as tolerated without significant pain. The leg lengths are equal and the range of motion at the ankle is 10/25 dorsiflexion/plantarflexion. He has mild pain over the ankle joint but no pain at the fracture site.
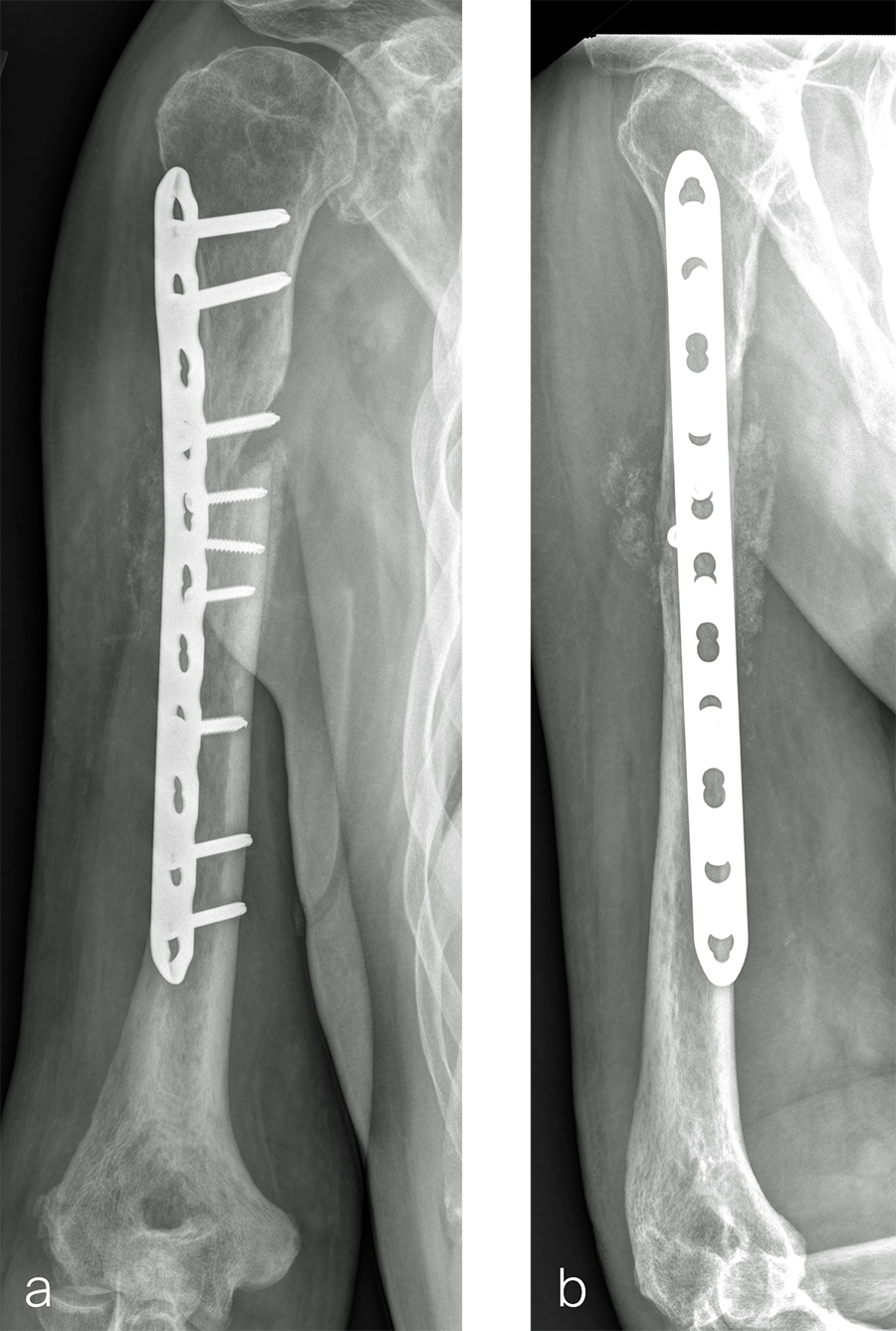
Case 2: RIA 2 use at the humerus (provided by Mark Lee, Sacramento, US)
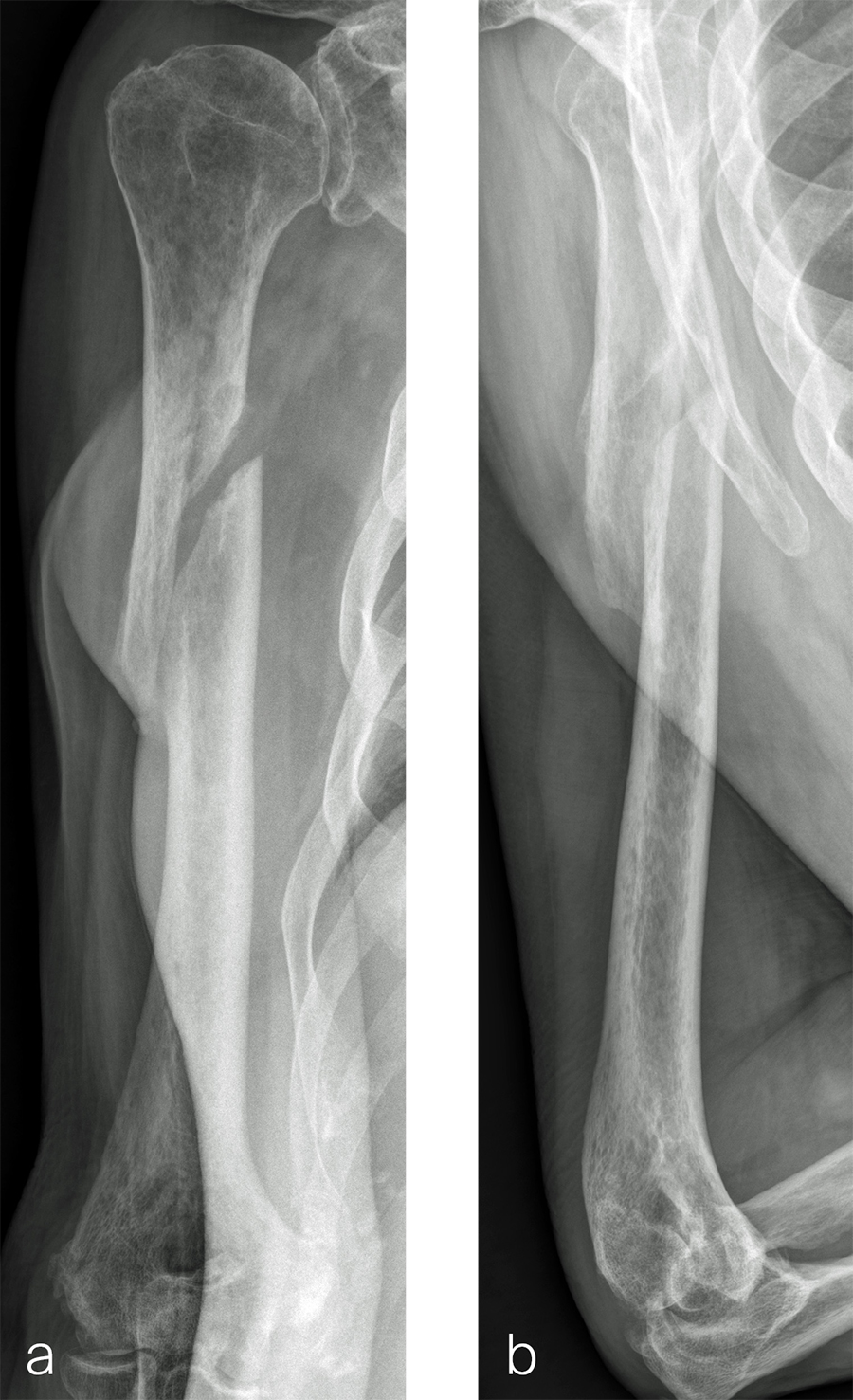
The patient was an 83-year-old right-hand dominant man who sustained a closed right humeral shaft fracture approximately 10 months before presentation. He was not initially offered surgical care and was treated with functional bracing. The patient has moderate pain but mainly complains of instability and lack of strength in his right arm. He was offered surgical nonunion repair with autogenous iliac crest bone grafting but declined iliac crest harvest. X-rays showed oblique nonunion with resorption and confirmed diagnosis of pseudarthrosis (Fig 7).
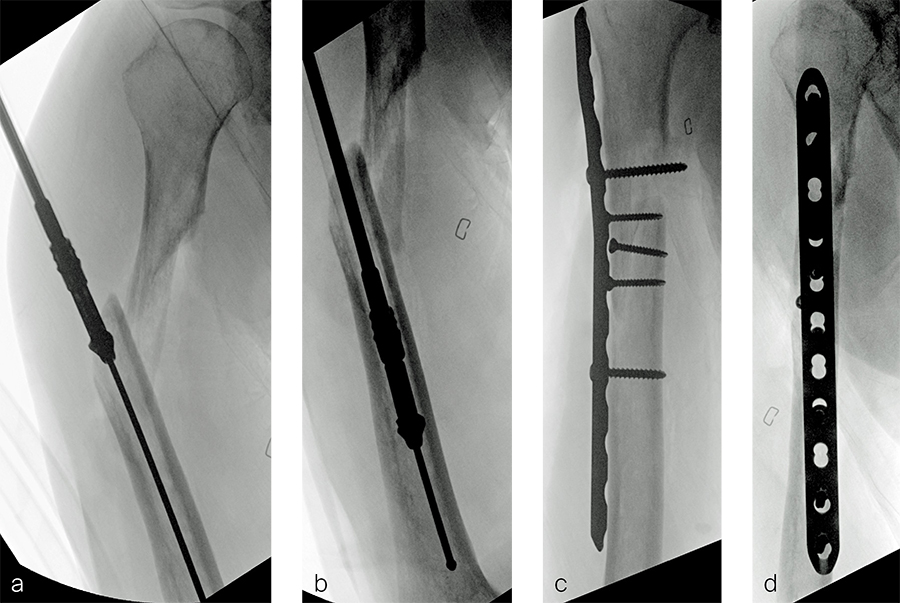
After debridement and resection of the pseudarthrosis, we accessed the distal segment of the humeral canal and used a small caliber RIA 2 reamer head to harvest intramedullary bone graft (Fig 8) for final plate fixation (Fig 9).
RIA 2 System: Next Generation Reamer-Irrigator-Aspirator
RIA 2 Surgical Technique Guide
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.