Variable Angle Locking Hand System
Douglas Campbell, Thomas Fischer
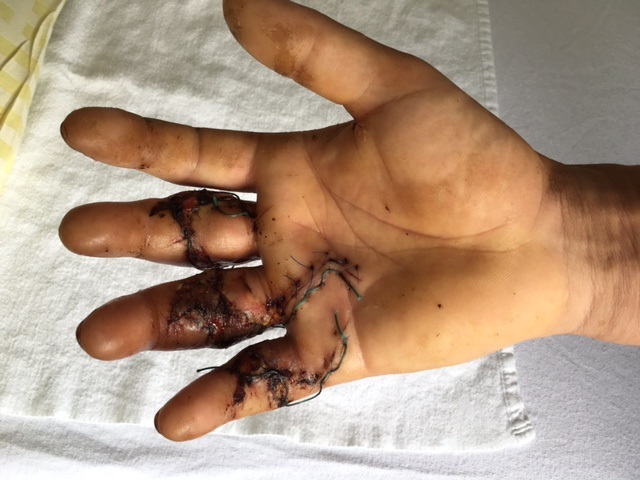
The AOTK approved Variable Angle Locking Hand System is the next generation of small implants for the hand consisting of plates that are anatomic, both fracture and procedure-specific and available in stainless steel and titanium. The system was designed to address clinical challenges faced in the current fixation of fractures and osteotomies and represents a complete overhaul of the LCP Compact Hand System. The Variable Angle Locking Hand System offers a solution for fracture fixation, arthrodesis, non-unions and the reconstruction of small bones and small bone fragments particularly in adults with osteopenic bone. The system also offers instrumentation to aid in fracture reduction, provisional fixation, implant adaptation and construct creation.
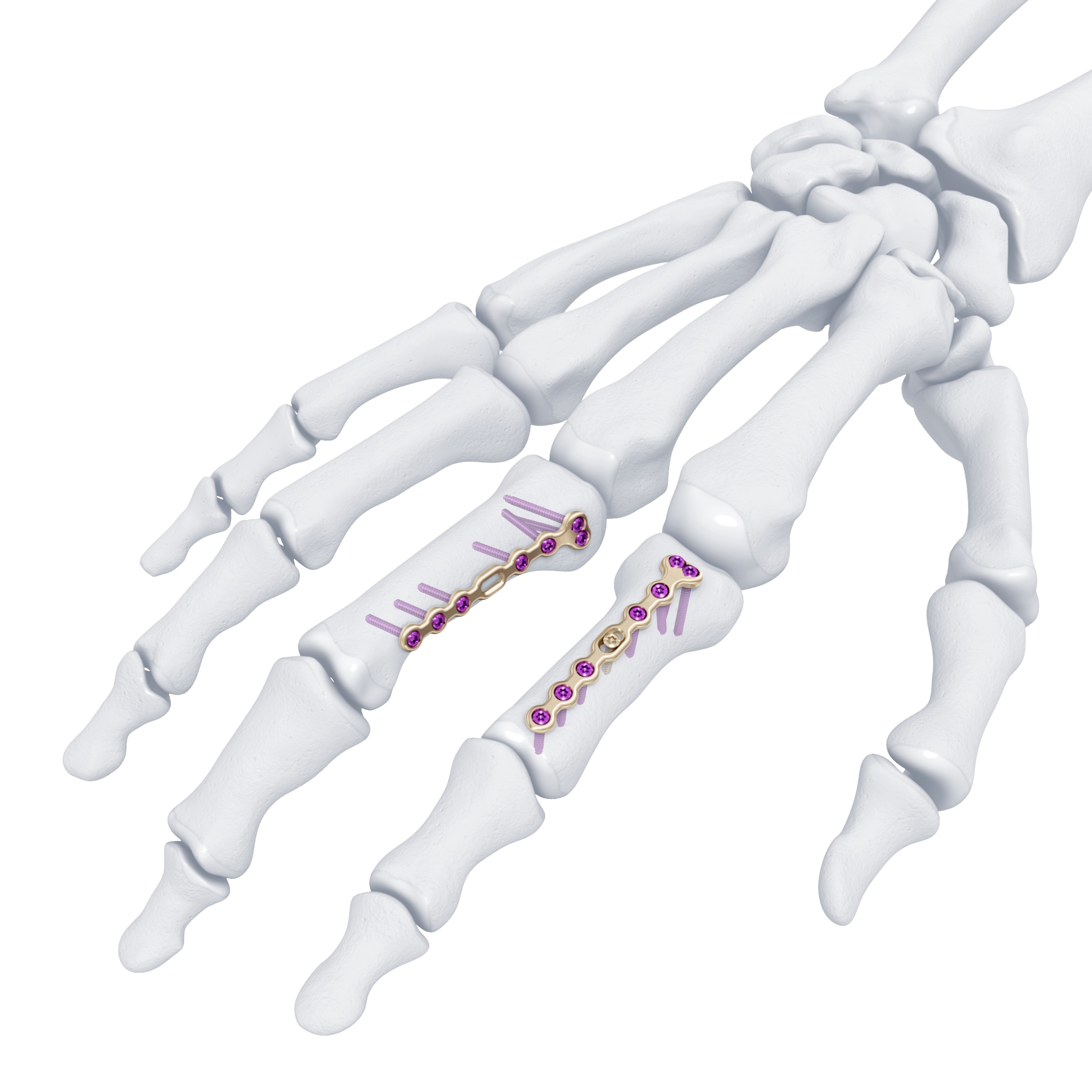
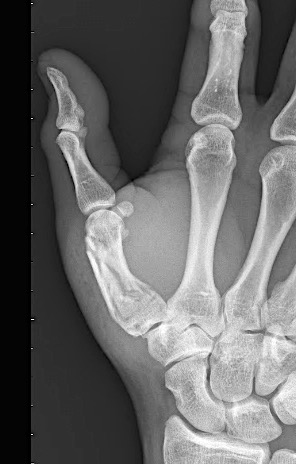
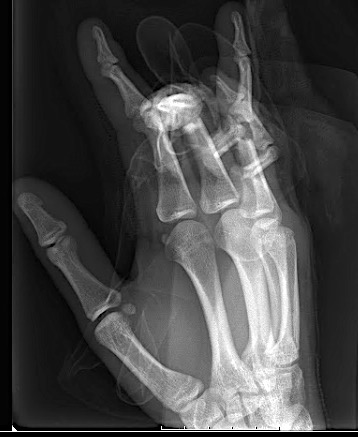
The previously approved Mini Fragment and Compact Hand System standard plates were designed to meet the needs of small bone fixation. The evolution of such internal fixation in the hand to now include variable angle technology (Fig 1) provides a perfect display of a variety of fixed angle devices that are more anatomically adapted with a lower profile design to accommodate the gliding structures of periarticular zones of the hand. The volume of implant beneath the extensor apparatus has been reduced both through plate design and the inclusion of smaller dimension implants (1.3 mm system).
The only fixed angle devices available in earlier systems were blade plate designs. In the new Variable Angle Locking Hand System, there exist a greater choice of implants which enable the application of axial stability and fixed-angle constructs in challenging fractures. Anatomically preformed plates also result in less bulk and a lower need for plate adaptation (Fig 2). A greater selection of fixed angle implants will allow for a wider range of application in osteopenic bone.
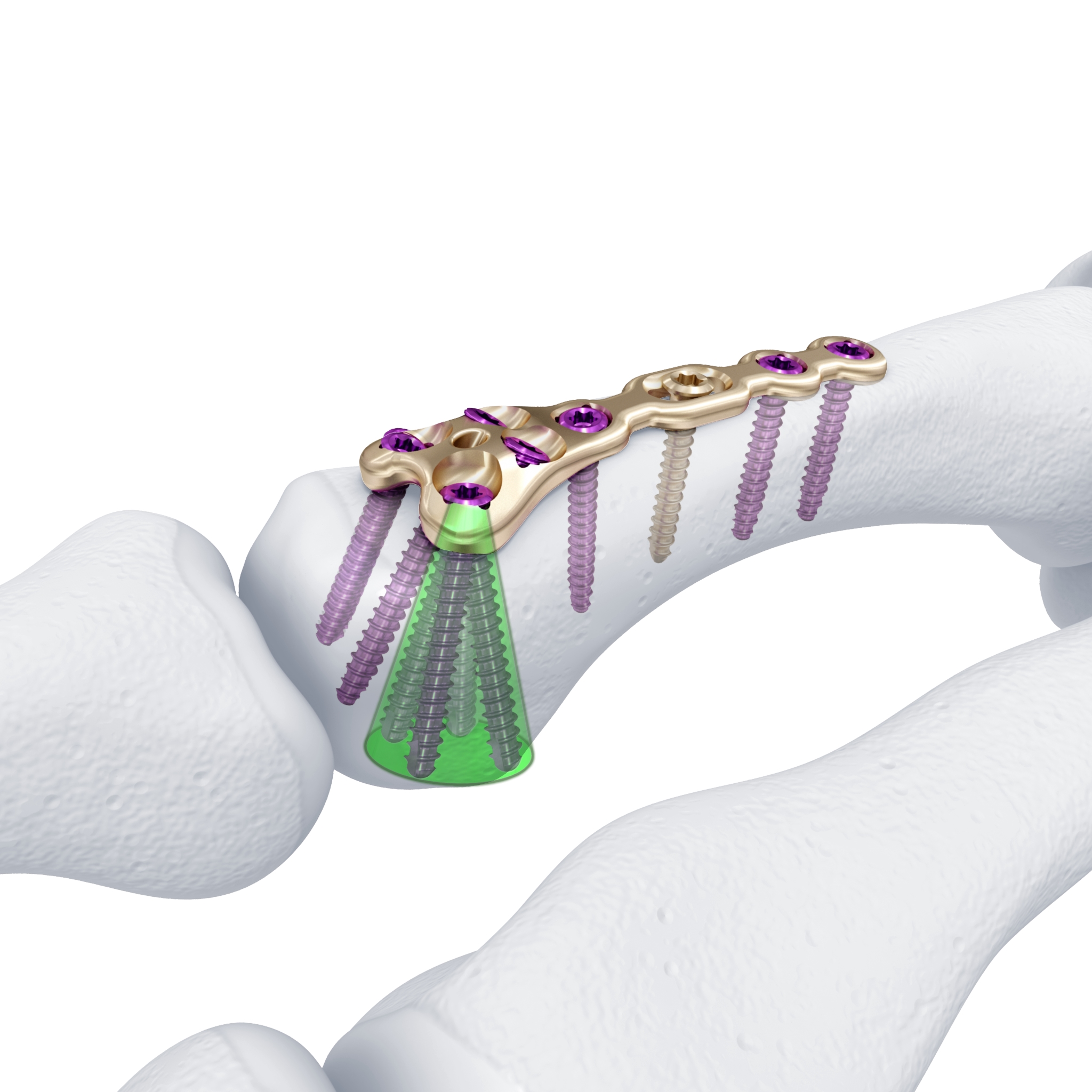
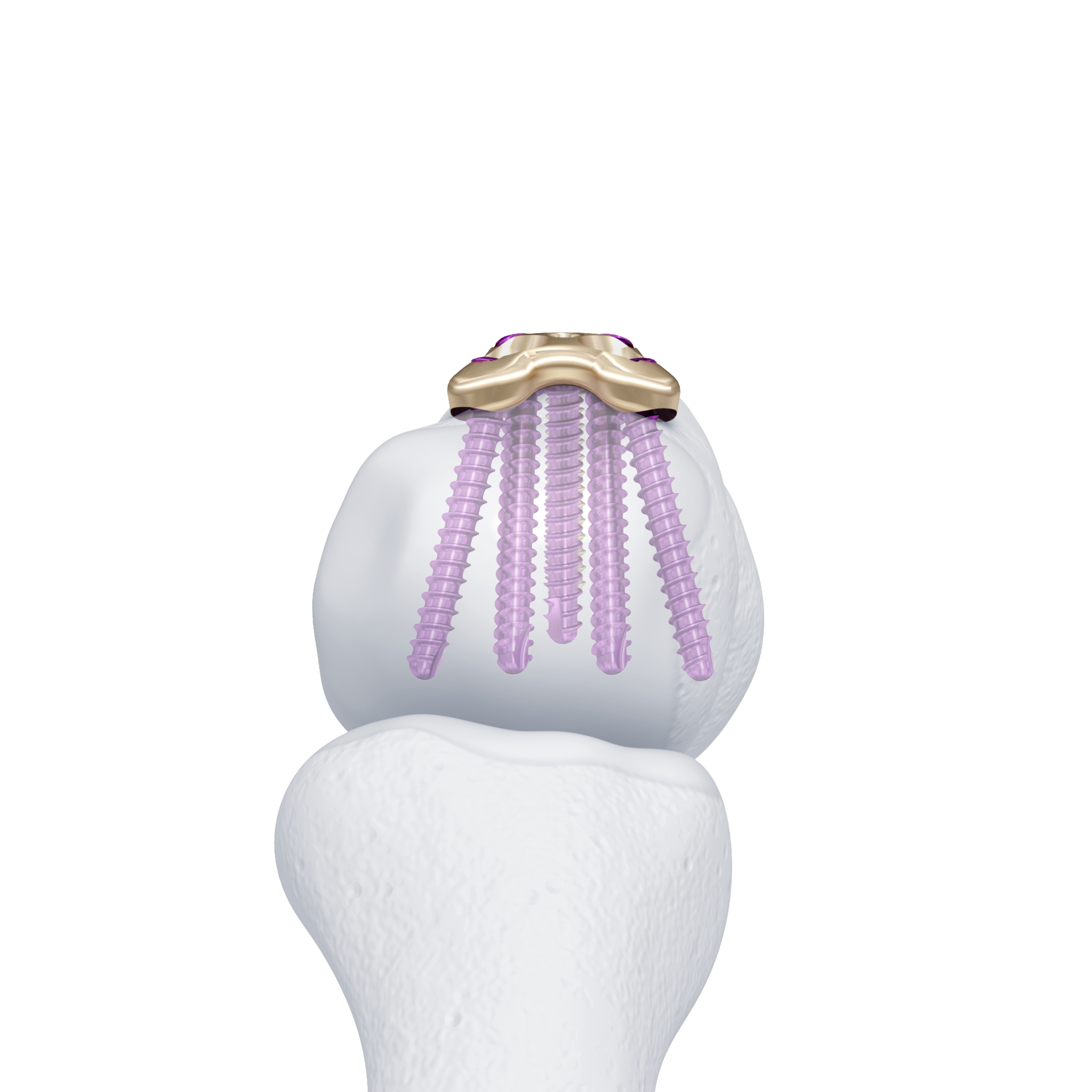
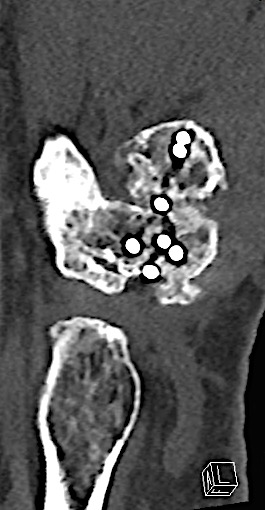
For reconstruction applications, a variety of implants that provide precise rotational adjustment have been added. Small rotational deformities of the digits are common and have a greater functional impact than other long bone deformities. These implants provide fine adjustment capabilities. Integration of variable angle locking technology (VA-LCP) into the 1.5 mm and 2.0 mm systems allow for improved placement of screws in metaphyseal bone. Safer and more accurate screw placement in periarticular and juxta-articular positions is realized with the VA-LCP (Fig 3).
Not only has the Variable Angle Locking Hand system delivered a significant advancement in implant development. The technology behind the instrumentation has also been advanced to overcome specific problems such as screwdriver retention (Fig 4), plate contouring and cutting and fracture reduction (Fig 5). The system includes improvements in the following areas:
- Provisional fixation
- Plate adaptation implant cutting and bending, better accuracy and surface/contour retention
- Refined bone preparation.
A comprehensive revitalization of the system was undertaken to provide implants that improved upon the teaching principles of the AO. Devices and designs were evaluated and improved to achieve stable fixation, anatomic reduction, accommodation of soft tissues and early functional aftercare. The Variable Angle Locking Hand System contains implants that extend the possibilities of stable fixation where none could be provided before (osteoporotic bone, small fragments).
This new system was developed to include the best practices in fracture care and to present a system that revolutionized both implant design and instrumentation in a complete overhaul of previous designs. Through a successful collaboration with the Hand Expert Group and numerous surgeons including AO faculty, the goal of creating a system that meets current daily clinical needs was achieved.
Cases provided by Esther Vögelin, Bern, Switzerland
Case 1:
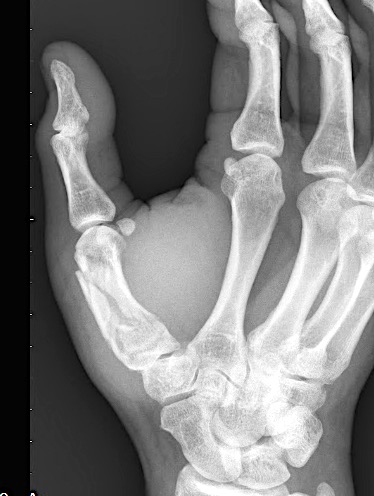
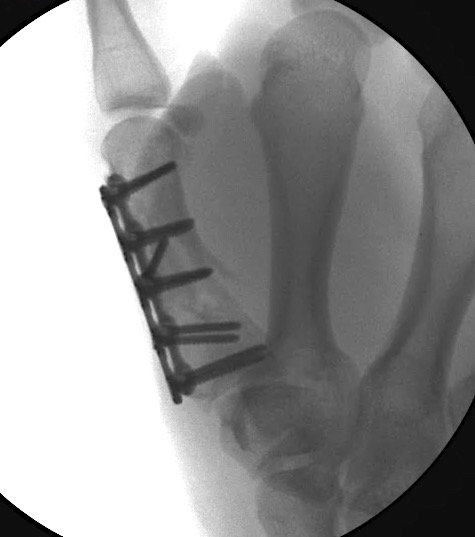
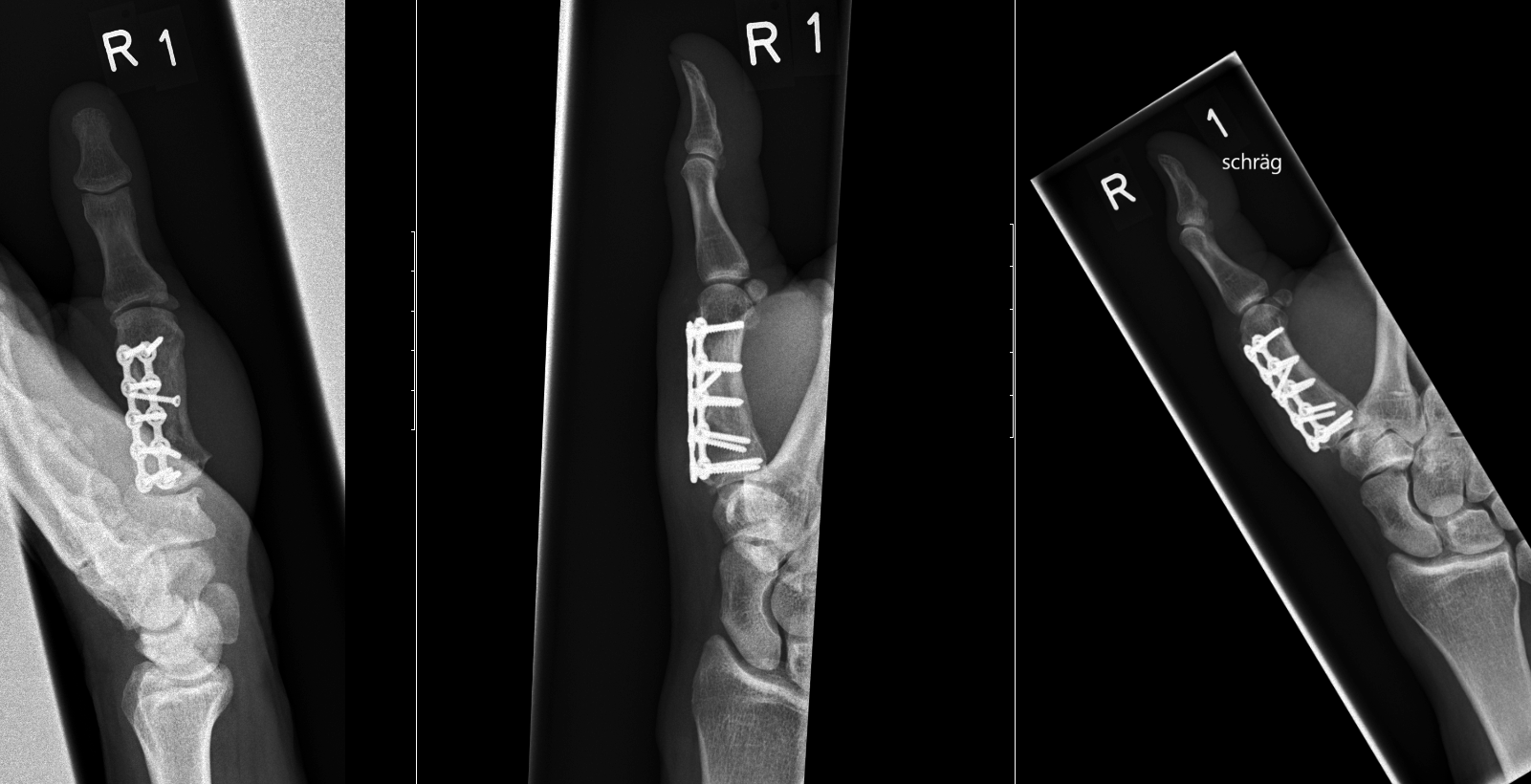
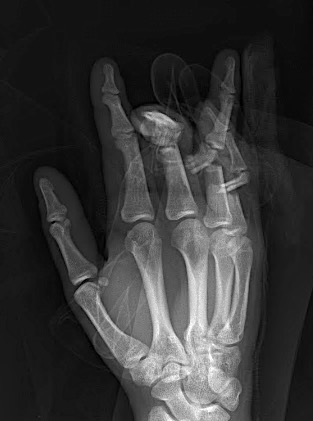
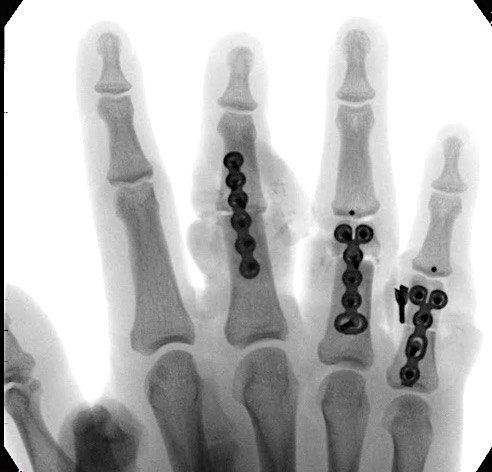
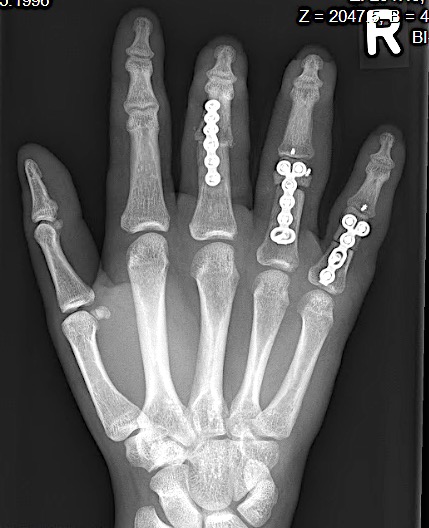
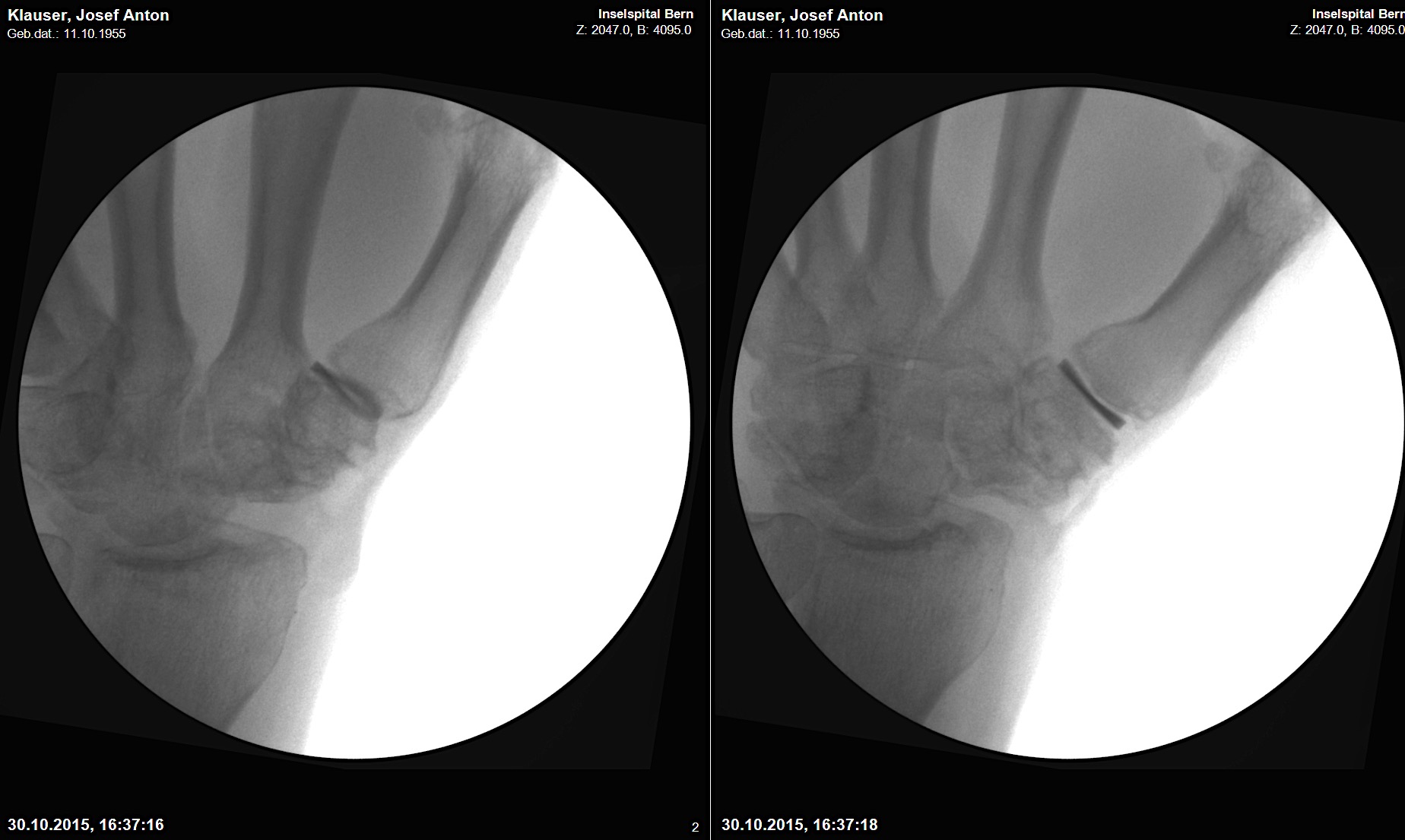
A 64-year-old male suffered a multi-fragmentary fracture of his right thumb metacarpal (Fig 1). An adapted 12-hole strut plate from the variable angle locking hand system was the implant of choice for fixation (Figs 2 - 4).
The strut plate provided good stability in a comminuted extraaricular fracture pattern and enables immediate mobilization. Bone callus formation was not witnessed during the healing process.
Case 2:
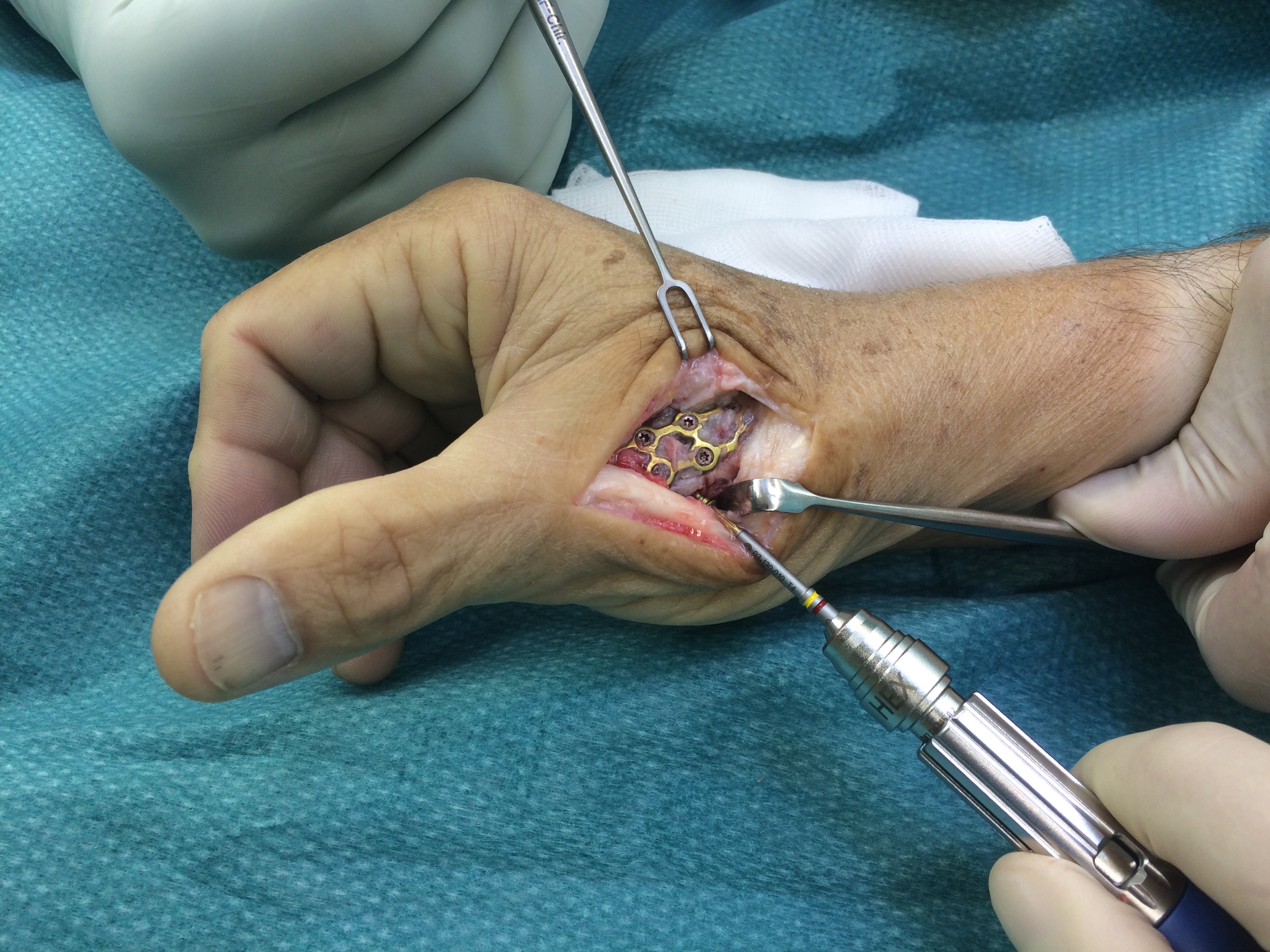
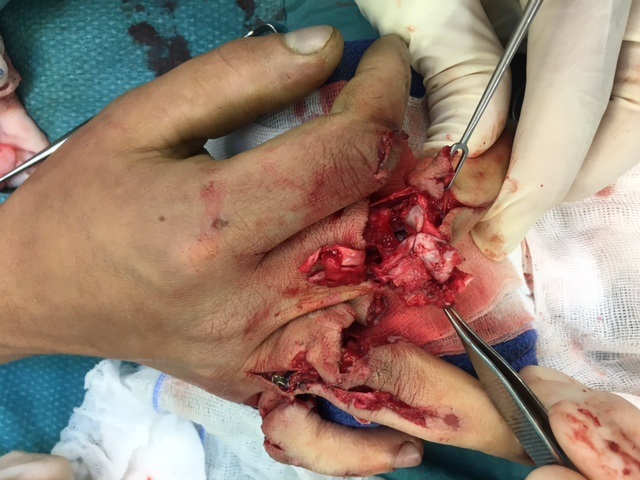
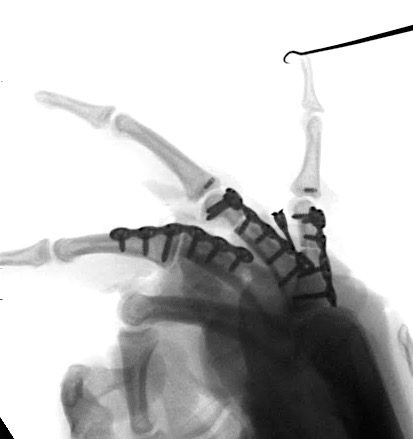
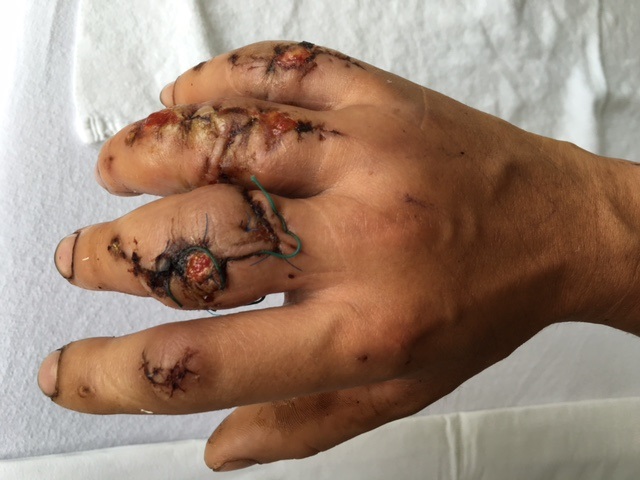
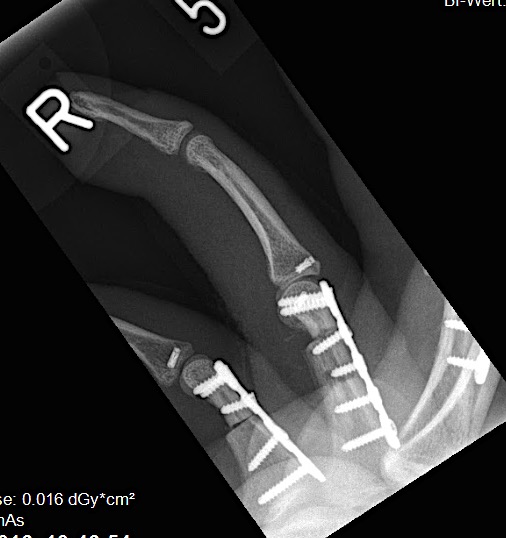
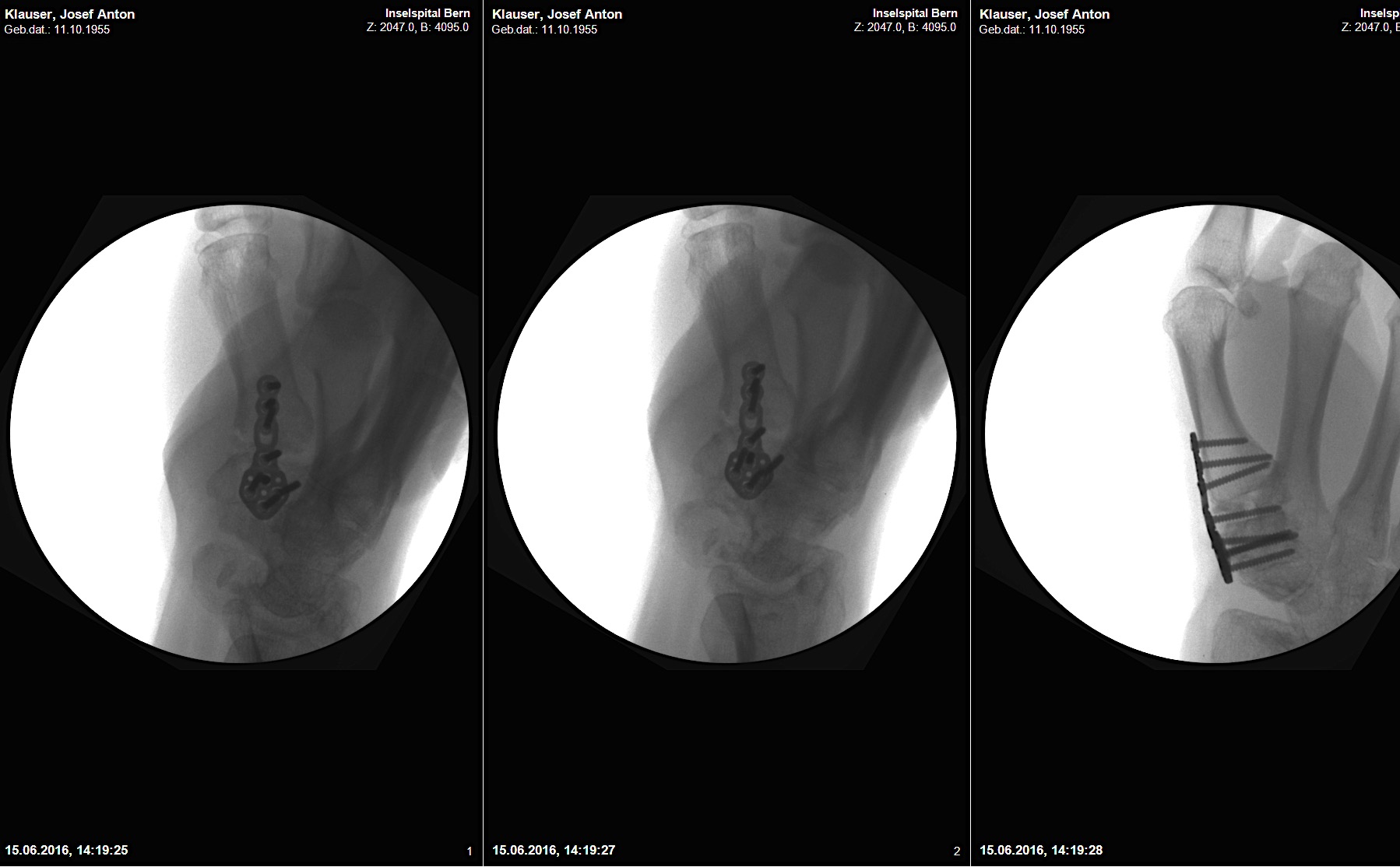
A 20-year-old male suffered a complex multi-digit injury of the right hand requiring revascularization and stabilization of both proximal phalanx and PIP joint fractures (Figs 5 - 8). Multiple plates, including the rotation correction plate from the 1.5 module of the VA Locking Hand System were used for fixation. The Variable Angle Locking system is ideal when only two screws, either proximal or distal, are able to be inserted due to space limitation.
One major advantage of variable angle technology in very distal phalangeal fractures is the ability to be extremely flexible with a wide range of fixation options. Freedom of implant placement assists early mobilization, vital in these complex fractures with associated soft-tissue trauma.
Case 3:
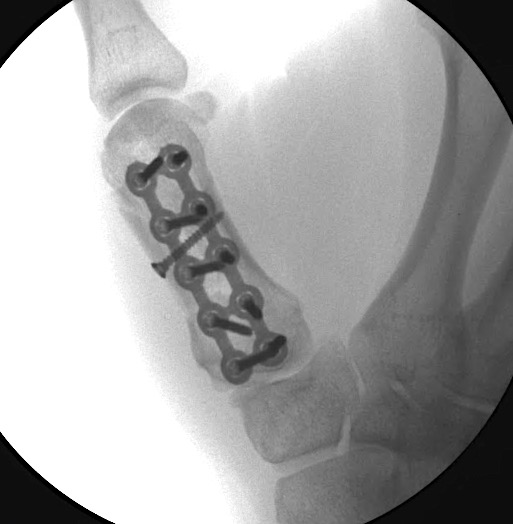
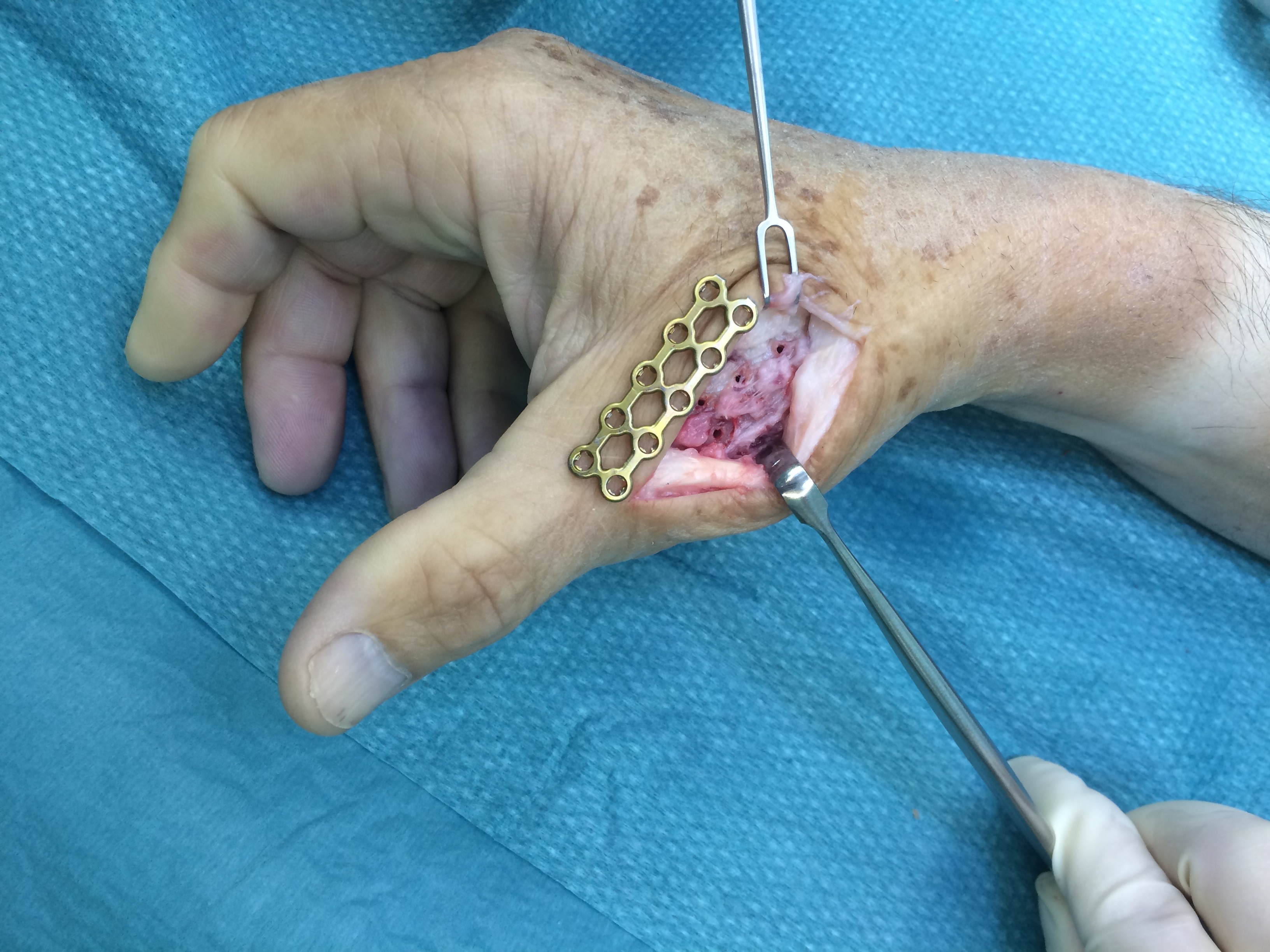
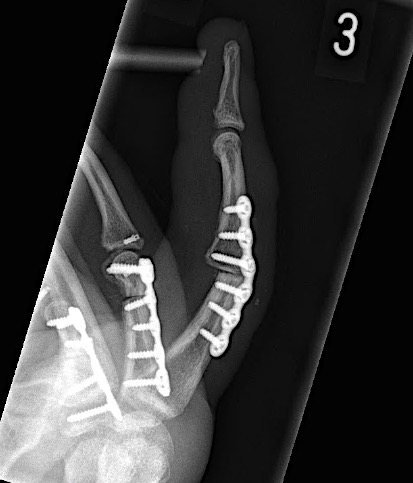
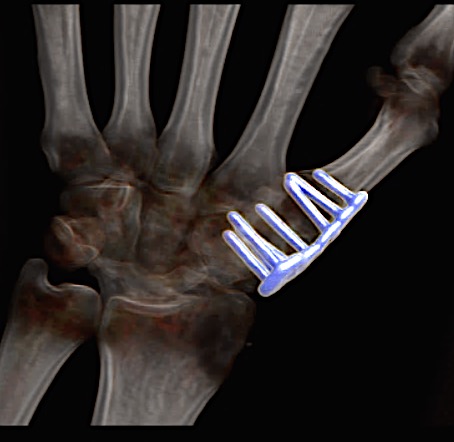
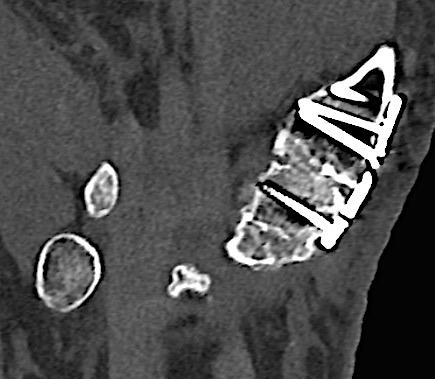
A 60-year-old male patient required an arthrodesis of the thumb carpometacarpal (CMC) joint after an implant arthroplasty in conjunction with the implantation of cortico-cancellous bone graft following a proximal row carpectomy and a previous arthrodesis between trapezium-trapezoid and the 2nd CMC joint (Fig 9). The first metacarpal dorsal plate from the VA Locking Hand System was selected for the procedure (Figs 10 - 11).
Introduction to the Variable Angle Modular Hand System
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.