LCP Pancarpal Arthrodesis Plate Family (2.7/3.5, 2.0/2.7, 2.0/2.4)
Erik Asimus, Brian Beale, Randy Boudrieau, Loïc Déjardin, Michael Kowaleski
Pancarpal arthrodesis is a surgical technique used as a salvage procedure for the treatment of carpal disorders including hyperextension injuries, severe fractures or luxations, degenerative joint disease, and congenital malformations in skeletally mature dogs. Despite its biomechanical disadvantage, the most common procedure relies on a dorsally applied bone plate for anatomical considerations. Complications include wound dehiscence, infection, failure of the central (radiocarpal) or distal screws, and fractures of the third metacarpal bone.
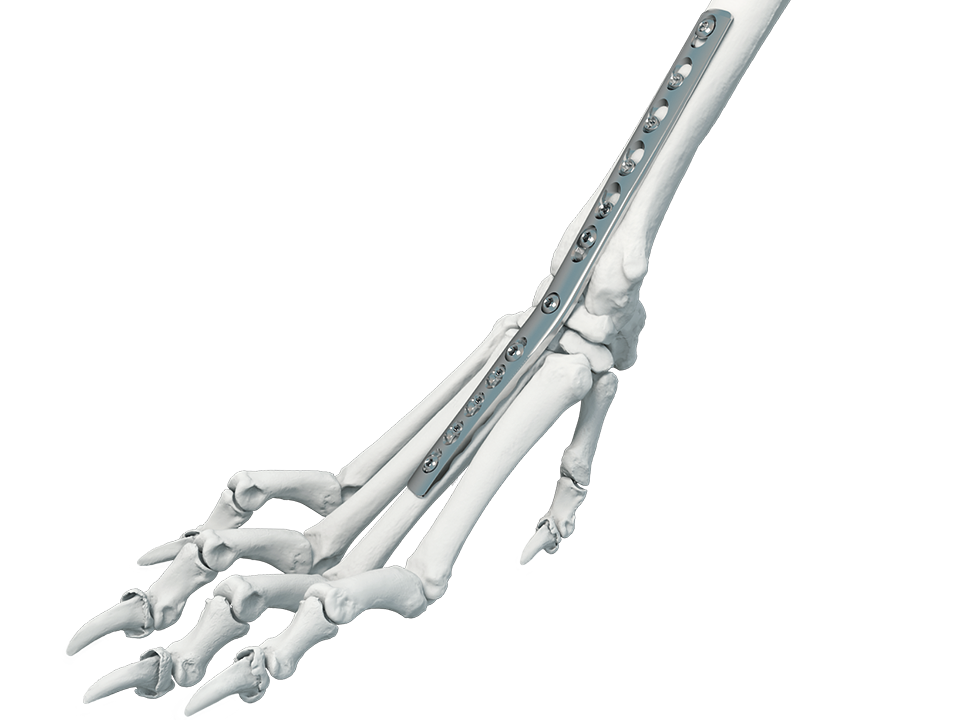
The recently launched 2.7/3.5 mm Pancarpal Arthrodesis (PCA) Plate (Fig 1) was designed specifically for carpal arthrodesis in skeletally mature dogs of 20-40 kg*.
Line Extension versions (2.0/2.7 & 2.0/2.4) have been specifically developed to fit the anatomy of smaller dogs.
Plate family overview
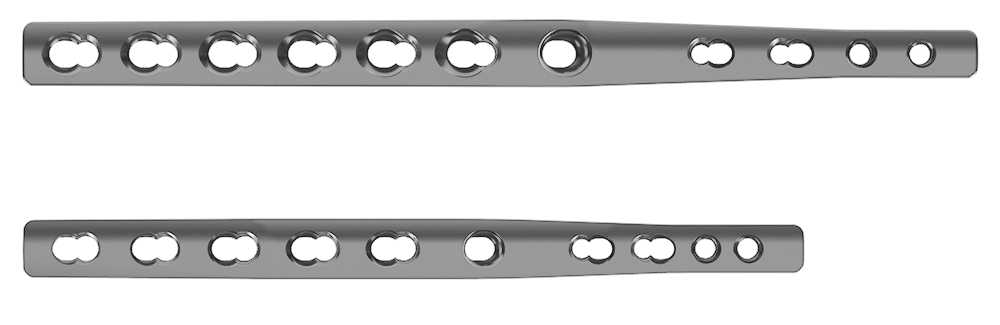
- 2.7/3.5 plate, 151 mm long, 3.3 mm thick, for dogs of 20-40 kg*
- 2.0/2.7 plate, 108 mm long, 2.6 mm thick, for dogs of 13-19 kg*
- 2.0/2.4 plate, 88 mm long, 2.0 mm thick, for dogs of 7-12 kg*
All Plates must be contoured to achieve an appropriate carpal extension angle; an angle of 15-20° is recommended.
Plate design
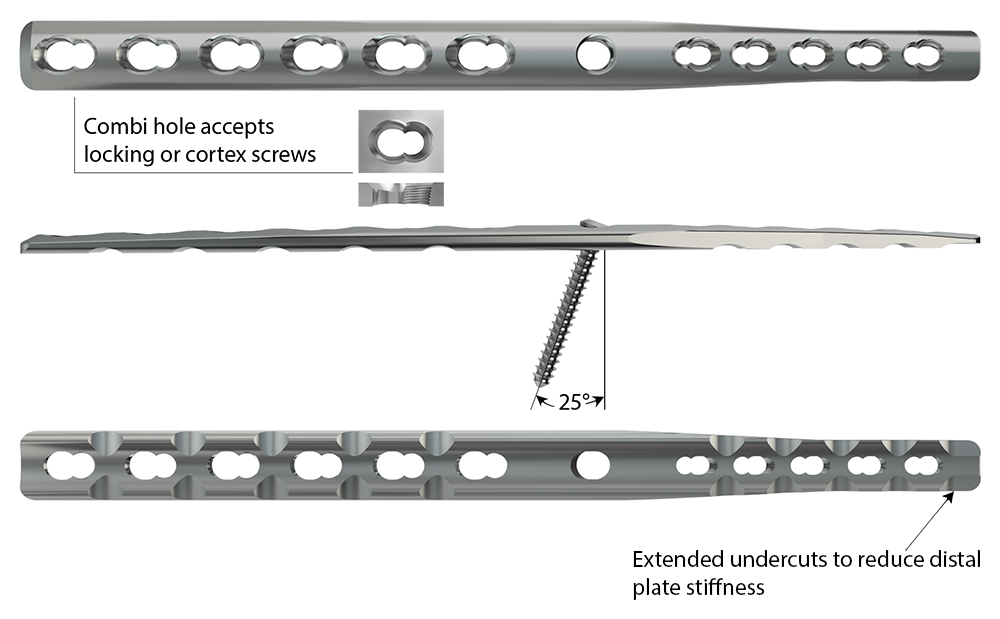
The following features are implemented in its design to mitigate the above-mentioned complications (Fig 2):
- Hybrid construction to occupy a smaller percentage of bone diameter in the third metacarpal bone
- Locking technology providing better stability and to preserve the bony vascular supply
- Combi-holes that accept either cortex or locking screws, and allow compression between radiocarpal, intercarpal, and carpometacarpal joints
- Optimized plate length with tapered thickness proximally and distally to accommodate soft-tissue closure and a reduced stiffness at the ends of the plate
- Center hole that provides up to 25° of proximal angulation and accepts a:
- 2.7 mm or 3.5 mm cortex screw (2.7/3.5 plate)
- 2.7 mm cortex screw (2.0/2.7 plate)
- 2.4 mm cortex screw (2.0/2.4 plate)
*Body weight indications for reference only. Other factors such as breed, skeletal anatomy and body score should be taken into consideration when selecting the proper implant.
Case 1: Hyperextension injury in a Labrador Retriever dog
(Case provided by Brian Beale, Houston, USA)
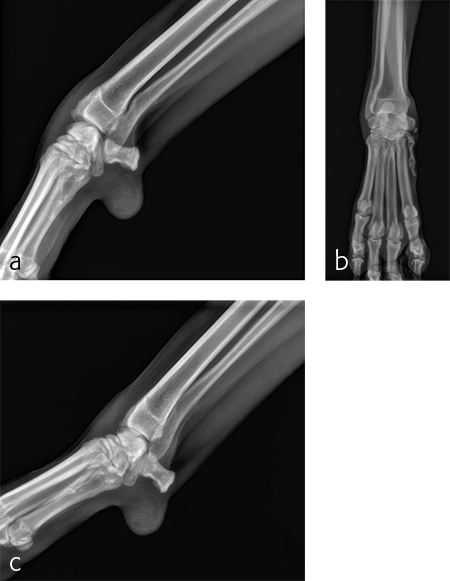
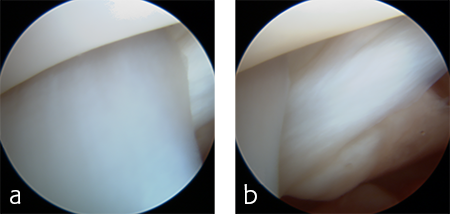
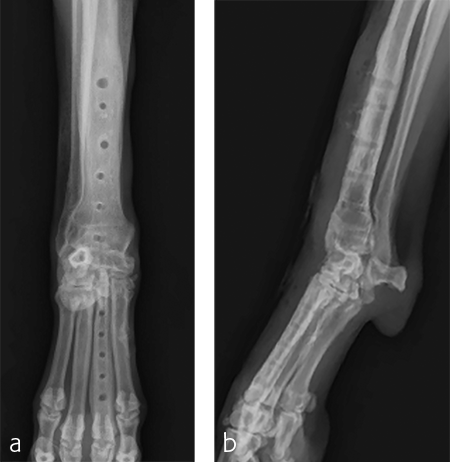
A 5-year-old neutered male Labrador Retriever weighing 24.6 kg presented with a right forelimb lameness of 4-month duration. The lameness occurred acutely and the dog was initially nonweight-bearing. The cause of the injury was not observed. Physical examination revealed a weight-bearing lameness of the right forelimb. The region of the right carpus was swollen compared to the left due to periarticular soft-tissue proliferation. A mild increase of synovial fluid and joint capsule distension was palpated. The right carpus was painful on extension and could be hyperextended to 30 degrees. The normal left carpus would extend to 10 degrees, which is the normal range of motion. Mild muscle atrophy of the right forelimb was observed. The neurological exam was normal. Radiographic examination revealed mild hyperextension, periarticular soft-tissue proliferation, and mild osteoarthritis (Fig 3). It was difficult to determine the joint level of instability of the right carpus based on the radiographic views. A diagnosis of hyperextension injury of the right carpus was made. The surgical plan included arthroscopic examination of the right carpus to assess the antebrachiocarpal joint to determine if it was affected, which would necessitate a pancarpal vs partial carpal arthrodesis.
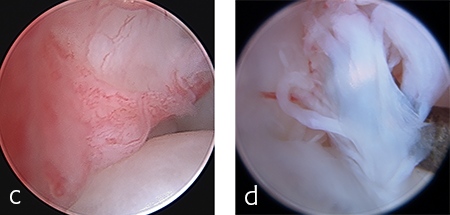
The patient was anesthetized and placed in ventral recumbency. Arthroscopic evaluation of the right antebrachiocarpal joint revealed synovitis and partial tearing of the palmar radiocarpal and ulnocarpal ligaments (Fig 4). A decision was made to perform a pancarpal arthrodesis due to arthroscopic evidence of damage at this level of the joint. A dorsal approach was made to the right carpus. The articular cartilage was debrided at all levels of the carpus. An autogenous cancellous bone graft was harvested form the right proximal humerus and was applied at the arthrodesis site.
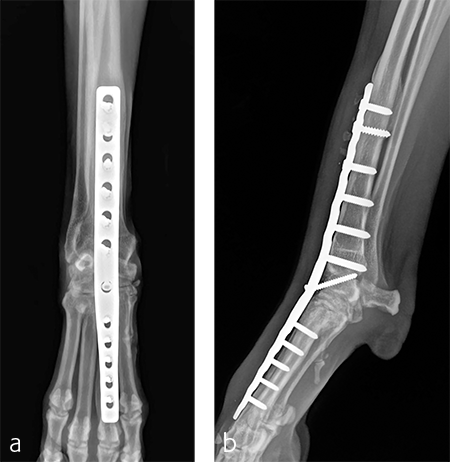
A 2.7/3.5 Pancarpal Arthrodesis was applied to the dorsal aspect of the carpus using a combination of locking and cortical screws. A 2.7 mm cortical screw was used to attach the plate to the radiocarpal bone. Five 2.7 mm locking screws were used to attach the plate to the 3rd metacarpal bone. Five 3.5 mm locking screws and one 3.5 mm cortical screw were used to attach the plate to the dorsal surface of the radius. A combination of locking and cortical screws was used to provide compression (cortical screw placed in eccentric position to provide compression across the radiocarpal joint) and achieve excellent stability and limb alignment. The incision was closed in routine fashion.
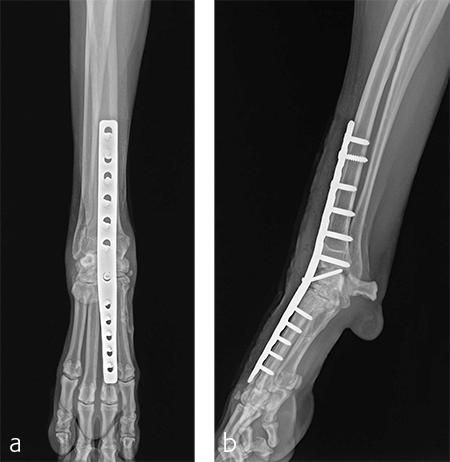
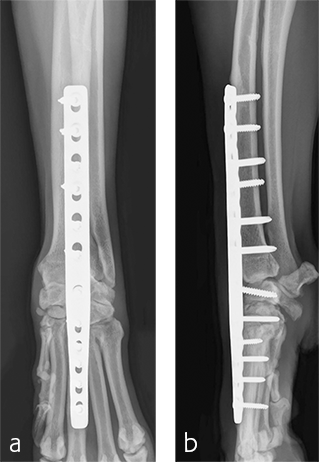
Postoperative radiographs revealed proper joint orientation and compression of the multiple antebrachiocarpal joint levels (Fig 5). Implant placement was considered excellent.
A custom fiberglass palmar splint was applied from the paw to just distal to the elbow after surgery. The splint was used for 4 weeks followed by a soft padded bandage for 4 weeks. Bandage changes were performed weekly. Activity was restricted to leash walks only for 12 weeks postoperatively. Radiographic examination 8 weeks after surgery revealed early healing of the pancarpal arthrodesis and stable implants. No complications were noted (Fig 6).
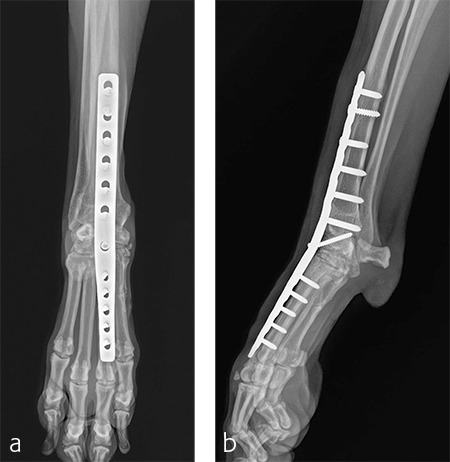
The patient was returned to normal activity 4 months after surgery. Radiographic examinations at 6 and 6.5 months after surgery revealed stable implants and fusion of the carpus (Fig 7 and 8). Functional outcome was excellent 1 year postoperatively. The patient had returned to full weight-bearing without lameness and the carpus was pain-free and stable.
Case 2: Traumatic hyperextension injury in a Labrador Retriever
(Case provided by Amy Kapatkin, Davis, USA)
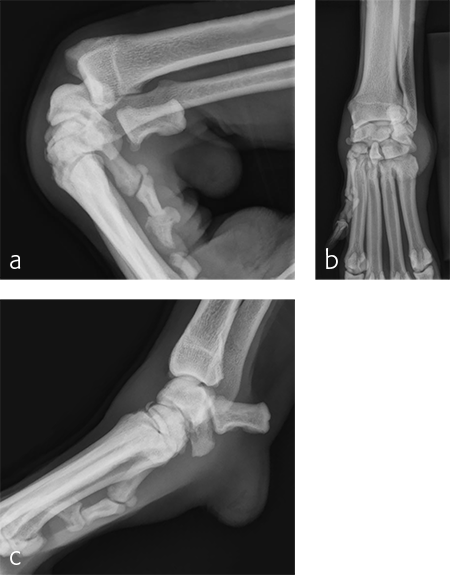
A 7-year-old, 32.5 kg, Labrador Retriever became acutely lame on the left thoracic limb while catching a ball. It was evaluated three weeks later and had carpal swelling, pain, and instability of the left carpus. Flexed lateral, craniocaudal, and extended mediolateral view images of the left carpus revealed a dorsal chip fracture at the carpometacarpal joint and hyperextension of the left carpus (Fig 9).
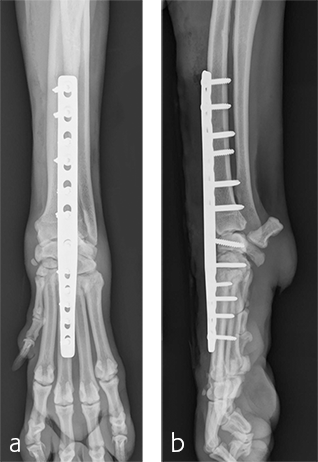
Treatment with a splint for several weeks resulted in no improvement. A pancarpal arthrodesis was performed with the 2.7/3.5 Pancarpal Arthrodesis Plate and a combination of standard cortical and locking screw fixation. An autogenous cancellous bone graft was collected from the left proximal humerus and placed at all joint levels.
Immediate postoperative images confirmed anatomic alignment and adequate carpal extension (Fig 10). At the 11-week postoperative follow-up examination, functional recovery was very good with images revealing stable implants and healing of the arthrodesis (Fig 11). The dog was then allowed to return to normal activity.
Pancarpal Arthrodesis Plate Family
Hazards and labeling
Due to varying countries’ legal and regulatory approval requirements, consult the appropriate local product labeling for approved intended use of the products described on this website. All devices on this website are approved by the AO Technical Commission. For logistical reasons, these devices may not be available in all countries worldwide at the date of publication.
Legal restrictions
This work was produced by AO Foundation, Switzerland. All rights reserved by AO Foundation. This publication, including all parts thereof, is legally protected by copyright.
Any use, exploitation or commercialization outside the narrow limits set forth by copyright legislation and the restrictions on use laid out below, without the publisher‘s consent, is illegal and liable to prosecution. This applies in particular to photostat reproduction, copying, scanning or duplication of any kind, translation, preparation of microfilms, electronic data processing, and storage such as making this publication available on Intranet or Internet.
Some of the products, names, instruments, treatments, logos, designs, etc referred to in this publication are also protected by patents, trademarks or by other intellectual property protection laws (eg, “AO” and the AO logo are subject to trademark applications/registrations) even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name, instrument, etc without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain.
Restrictions on use: The rightful owner of an authorized copy of this work may use it for educational and research purposes only. Single images or illustrations may be copied for research or educational purposes only. The images or illustrations may not be altered in any way and need to carry the following statement of origin “Copyright by AO Foundation, Switzerland”.
Check www.aofoundation.org/disclaimer for more information.
If you have any comments or questions on the articles or the new devices, please do not hesitate to contact us.
“approved by AO Technical Commission” and “approved by AO”
The brands and labels “approved by AO Technical Commission” and “approved by AO”, particularly "AO" and the AO logo, are AO Foundation's intellectual property and subject to trademark applications and registrations, respectively. The use of these brands and labels is regulated by licensing agreements between AO Foundation and the producers of innovation products obliged to use such labels to declare the products as AO Technical Commission or AO Foundation approved solutions. Any unauthorized or inadequate use of these trademarks may be subject to legal action.
AO ITC Innovations Magazine
Find all issues of the AO ITC Innovations Magazine for download here.
Innovation Awards
Recognizing outstanding achievements in development and fostering excellence in surgical innovation.